Which Combination Of Atoms Can Form A Polar Covalent Bond
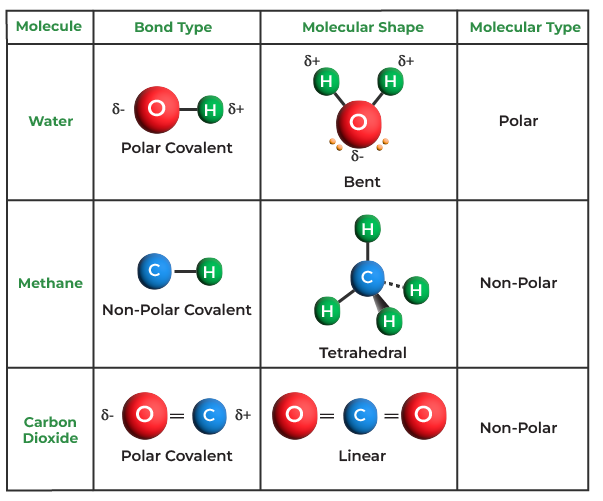
Which Combination Of Atoms Can Form A Polar Covalent Bond - A polar covalent bond is created when the shared electrons between atoms are not equally shared. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. This occurs when one atom has a higher. Covalent bonding occurs when pairs of electrons are shared by atoms. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. Only h and br form polar covalent bond. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a.
एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. This occurs when one atom has a higher. Covalent bonding occurs when pairs of electrons are shared by atoms. Only h and br form polar covalent bond. A polar covalent bond is created when the shared electrons between atoms are not equally shared.
As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Only h and br form polar covalent bond. This occurs when one atom has a higher. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Covalent bonding occurs when pairs of electrons are shared by atoms. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar.
Definition and Examples of a Polar Bond
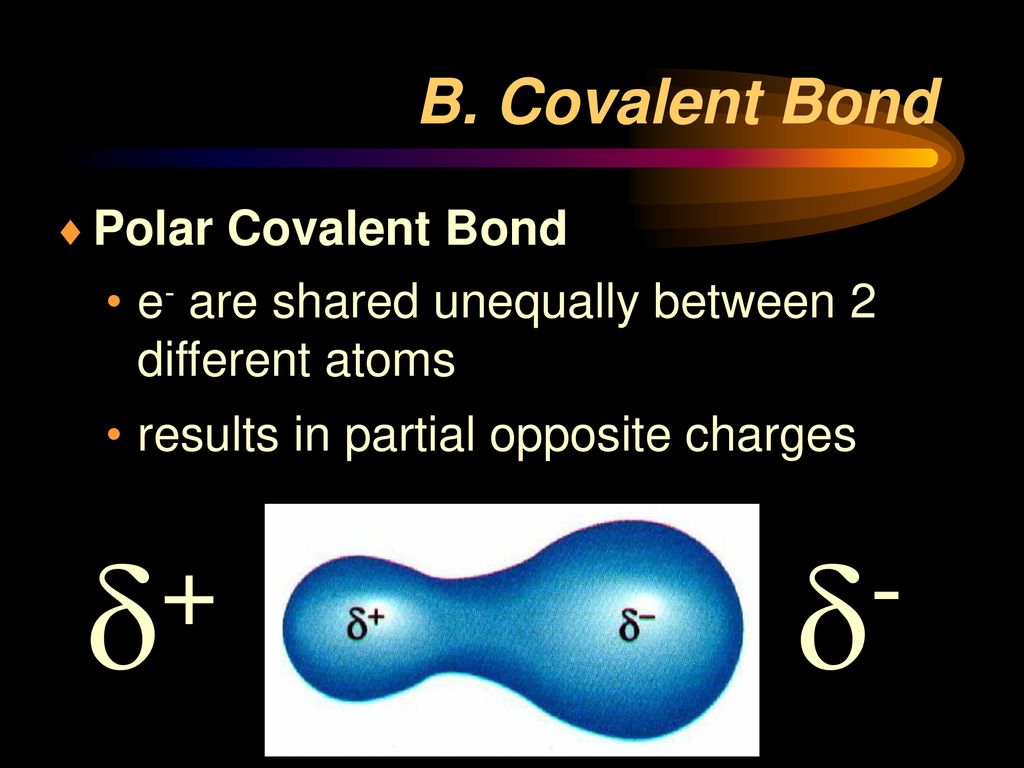
A polar covalent bond is created when the shared electrons between atoms are not equally shared. Only h and br form polar covalent bond. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Covalent bonding occurs when pairs of electrons are shared by atoms. This occurs when one atom has.
Covalent Bond Definition and Examples
A polar covalent bond is created when the shared electrons between atoms are not equally shared. This occurs when one atom has a higher. Only h and br form polar covalent bond. Covalent bonding occurs when pairs of electrons are shared by atoms. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो.
Polar Covalent Bond Definition And Examples, 49 OFF
Only h and br form polar covalent bond. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. Covalent bonding occurs when pairs of electrons are shared.
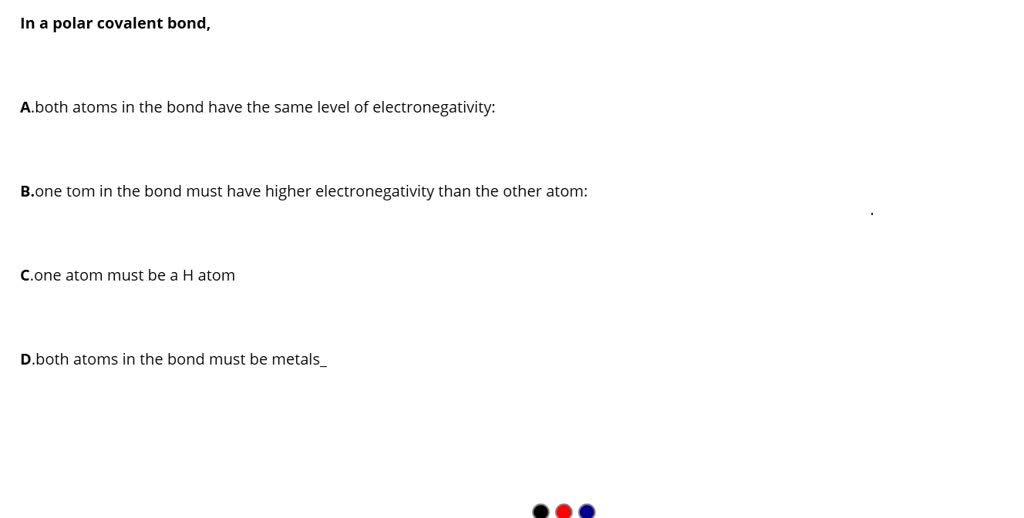
SOLVED In a polar covalent bond, A) both atoms in the bond have the
As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Only h and br form polar covalent bond. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Explain why the covalent bonds between , oxygen.
Polar Covalent Bonds Clearly Explained for Easy Learning
एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. A polar covalent bond is created when the shared electrons between atoms are not equally shared. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Only h and br form polar covalent bond. Covalent bonding occurs when pairs of electrons are.
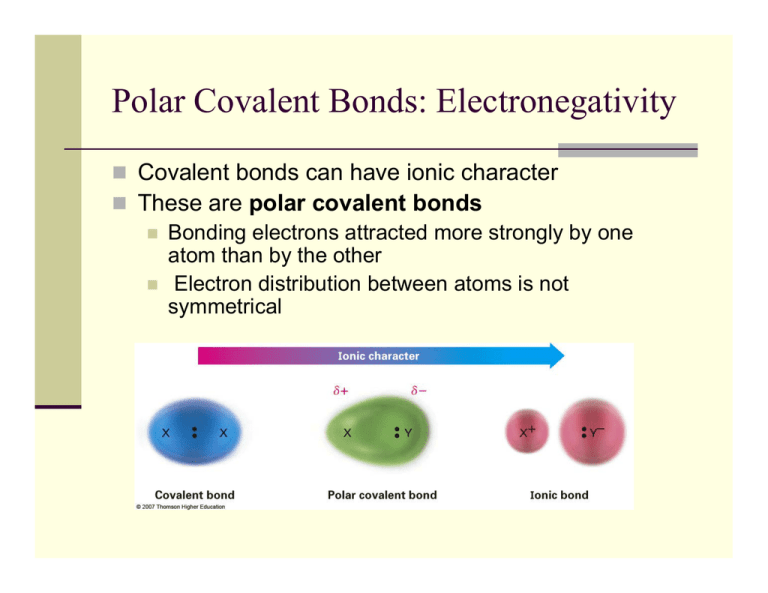
Polar Covalent Bonds Electronegativity
Covalent bonding occurs when pairs of electrons are shared by atoms. This occurs when one atom has a higher. Only h and br form polar covalent bond. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. A polar covalent bond is created when the shared electrons between atoms are not.
Reading Covalent Bonds Biology I
A polar covalent bond is created when the shared electrons between atoms are not equally shared. This occurs when one atom has a higher. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Explain why the covalent bonds between , oxygen.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
A polar covalent bond is created when the shared electrons between atoms are not equally shared. Covalent bonding occurs when pairs of electrons are shared by atoms. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. This occurs when one atom has a higher. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule.
Solved Question 1 (1 point)A polar covalent bond would form
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. This occurs when one atom has a higher. A polar covalent bond is created when the shared electrons between atoms are not equally shared. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Covalent bonding occurs when pairs of electrons are shared.
II. Kinds of Chemical Bonds Ionic Bond Covalent Bond Comparison Chart
Covalent bonding occurs when pairs of electrons are shared by atoms. A polar covalent bond is created when the shared electrons between atoms are not equally shared. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Only h and br form.
This Occurs When One Atom Has A Higher.
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Covalent bonding occurs when pairs of electrons are shared by atoms. Only h and br form polar covalent bond.
एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो.
A polar covalent bond is created when the shared electrons between atoms are not equally shared.
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)