What Type Of Electron Is Available To Form Bonds
What Type Of Electron Is Available To Form Bonds - Atoms form chemical bonds to achieve a full outer energy level, which. Bonds form when atoms share or transfer valence electrons. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Chemical bonding tends to be of two types;
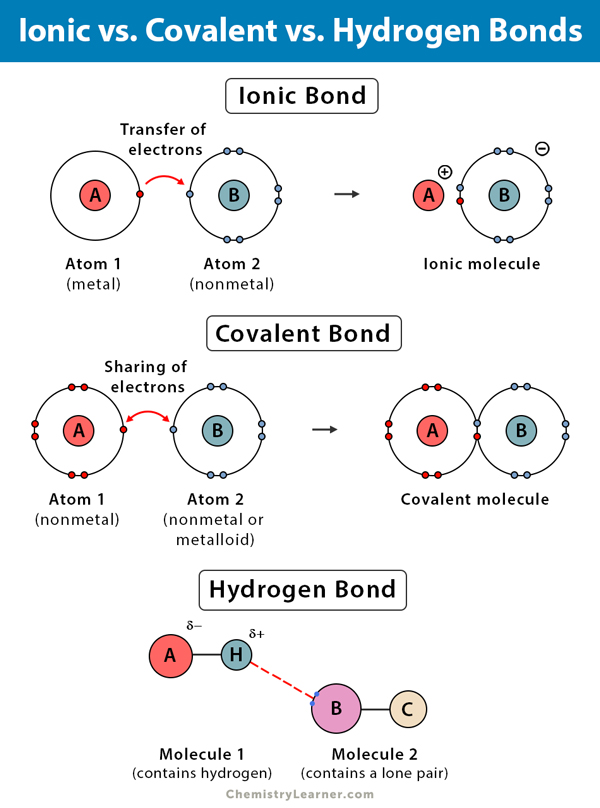
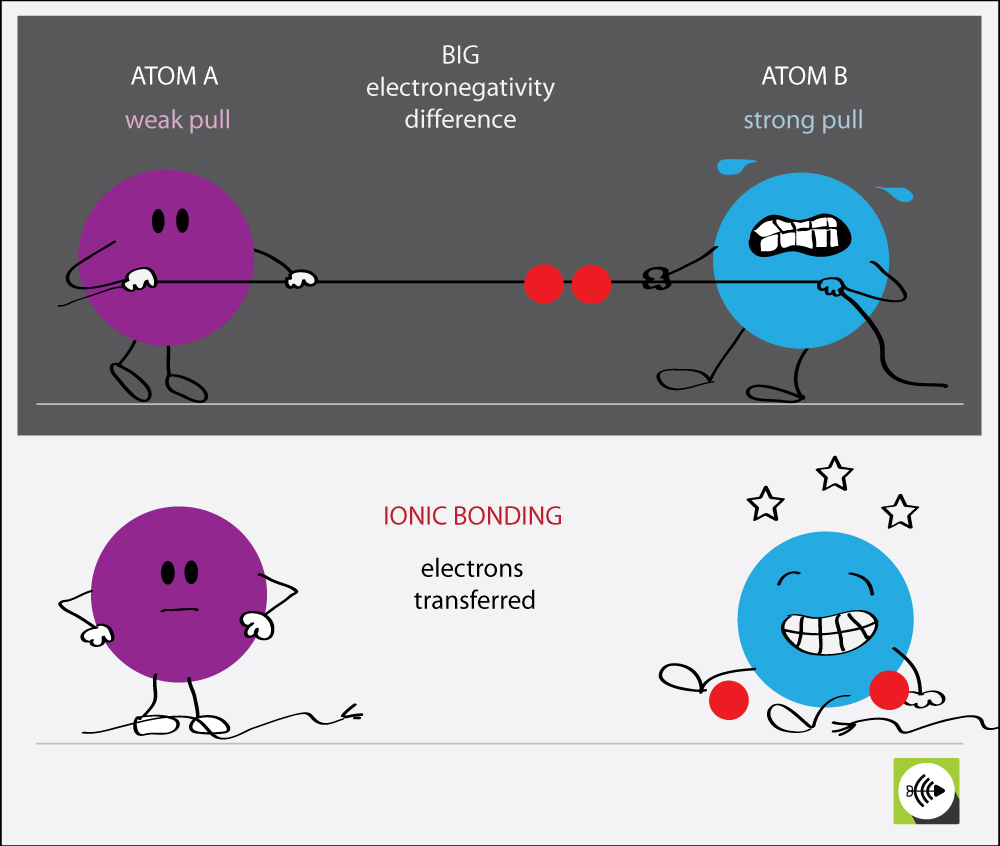
Bonds form when atoms share or transfer valence electrons. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Chemical bonding tends to be of two types; Atoms form chemical bonds to achieve a full outer energy level, which.
Bonds form when atoms share or transfer valence electrons. Atoms form chemical bonds to achieve a full outer energy level, which. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Chemical bonding tends to be of two types;
All organic molecules contain which element? ppt download
Chemical bonding tends to be of two types; Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Atoms form chemical bonds to achieve a full outer energy level, which. Bonds form when atoms share or transfer valence electrons.
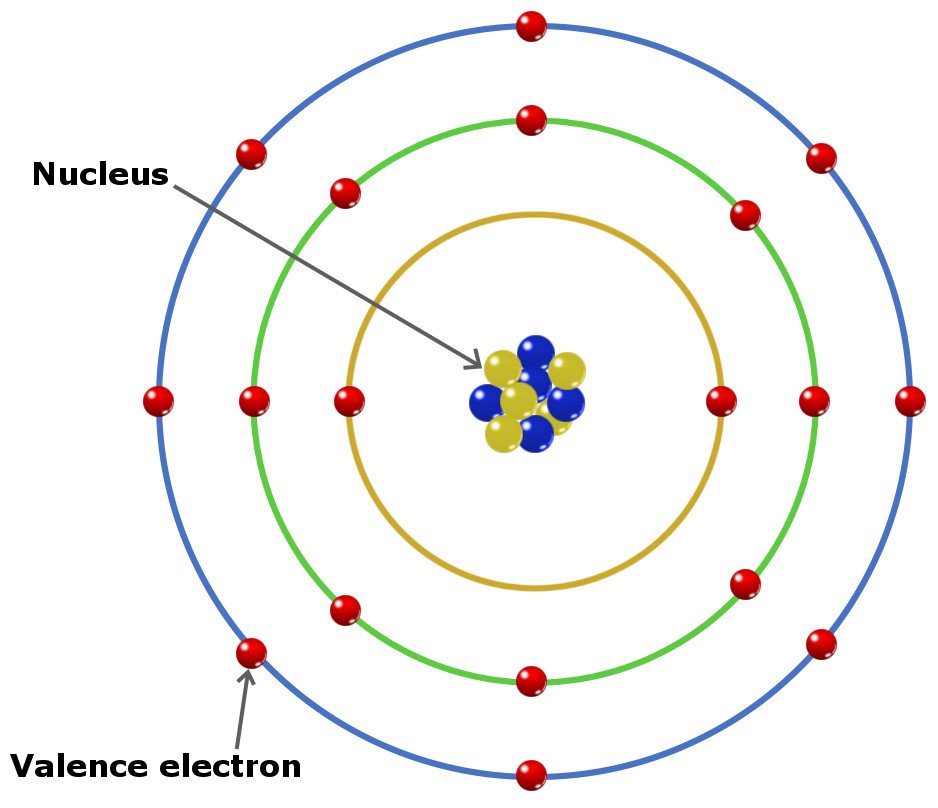
What Are Valence Electrons And How To Find Them? Where Are They Located?
Chemical bonding tends to be of two types; Atoms form chemical bonds to achieve a full outer energy level, which. Bonds form when atoms share or transfer valence electrons. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely.
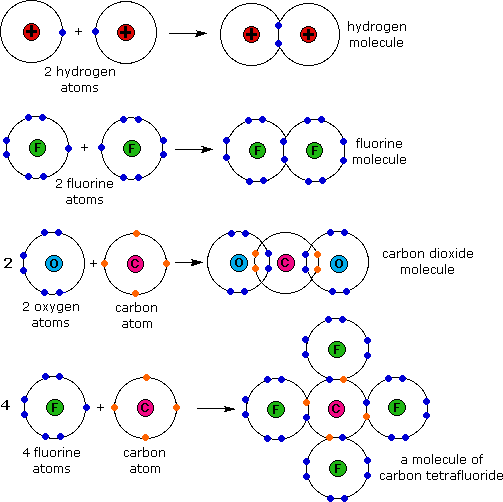
Covalent Bond sharing of electrons between atoms; bonds contain energy
Atoms form chemical bonds to achieve a full outer energy level, which. Chemical bonding tends to be of two types; Bonds form when atoms share or transfer valence electrons. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely.
Hydrogen Bond Definition, Types, and Examples
Bonds form when atoms share or transfer valence electrons. Atoms form chemical bonds to achieve a full outer energy level, which. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Chemical bonding tends to be of two types;
What Are Valence Electrons? Definition and Periodic Table
Chemical bonding tends to be of two types; Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Atoms form chemical bonds to achieve a full outer energy level, which. Bonds form when atoms share or transfer valence electrons.
Covalent Bonding (Biology) — Definition & Role Expii
Chemical bonding tends to be of two types; Bonds form when atoms share or transfer valence electrons. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Atoms form chemical bonds to achieve a full outer energy level, which.
Expanded Electron Configuration of Chlorine Womack Thille
Atoms form chemical bonds to achieve a full outer energy level, which. Chemical bonding tends to be of two types; Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Bonds form when atoms share or transfer valence electrons.
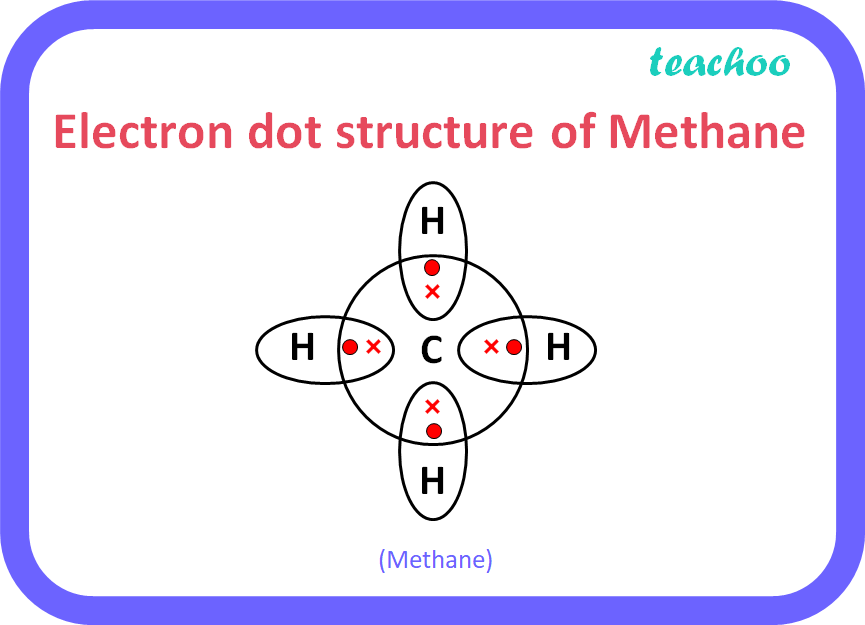
What is methane? Draw its electron dot structure. Name type of bonds
Chemical bonding tends to be of two types; Atoms form chemical bonds to achieve a full outer energy level, which. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Bonds form when atoms share or transfer valence electrons.
What type of electron is available to form bonds?
Chemical bonding tends to be of two types; Bonds form when atoms share or transfer valence electrons. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Atoms form chemical bonds to achieve a full outer energy level, which.
Electronegativity Bond Scale Surfguppy Chemistry made easy for
Covalent, in which electrons are shared between atoms, and ionic in which two oppositely. Atoms form chemical bonds to achieve a full outer energy level, which. Chemical bonding tends to be of two types; Bonds form when atoms share or transfer valence electrons.
Chemical Bonding Tends To Be Of Two Types;
Atoms form chemical bonds to achieve a full outer energy level, which. Bonds form when atoms share or transfer valence electrons. Covalent, in which electrons are shared between atoms, and ionic in which two oppositely.