What Is The Hybridization Of The Central Atom In Nh3
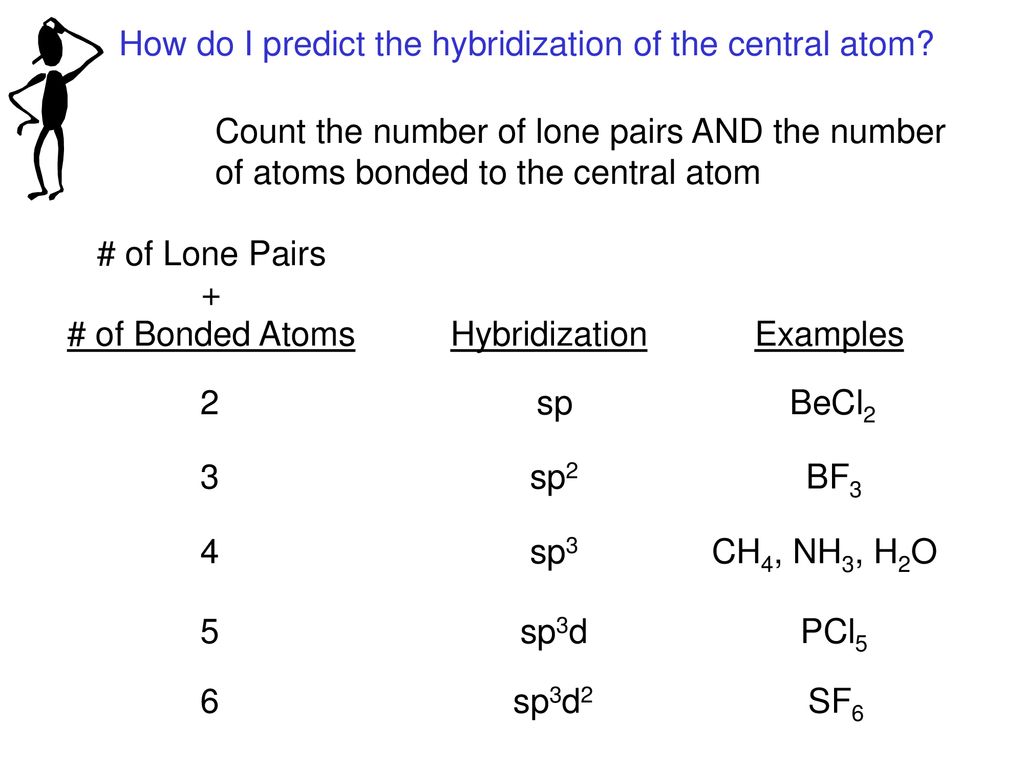
What Is The Hybridization Of The Central Atom In Nh3 - The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
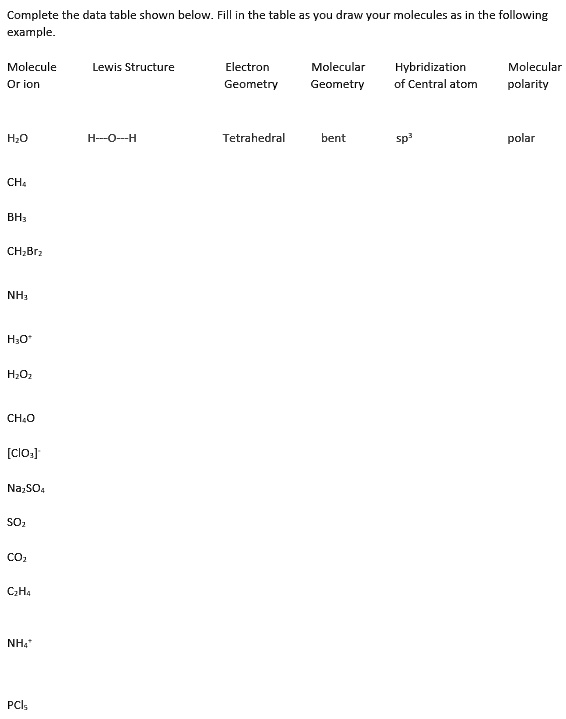
SOLVED Complete the data table shown below. Fill the table example
Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. We'll look at how to figure out if nh 3 is.
Experiments show O2 is ppt download
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
What is the hybridization of the central atom in NH3? Hybridization
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
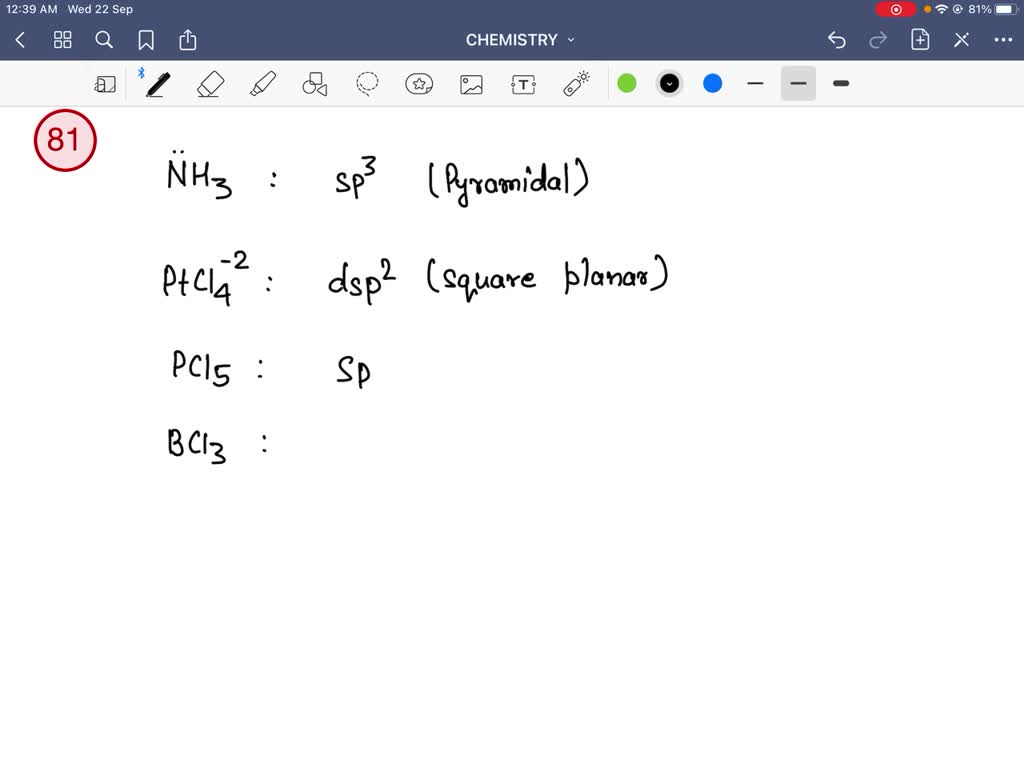
The correct order of hybridization of the central atom in the following
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
Hybridization and Hybrid Orbitals ChemTalk
We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³.
The correct order of hybridization of the central atom in the following
We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
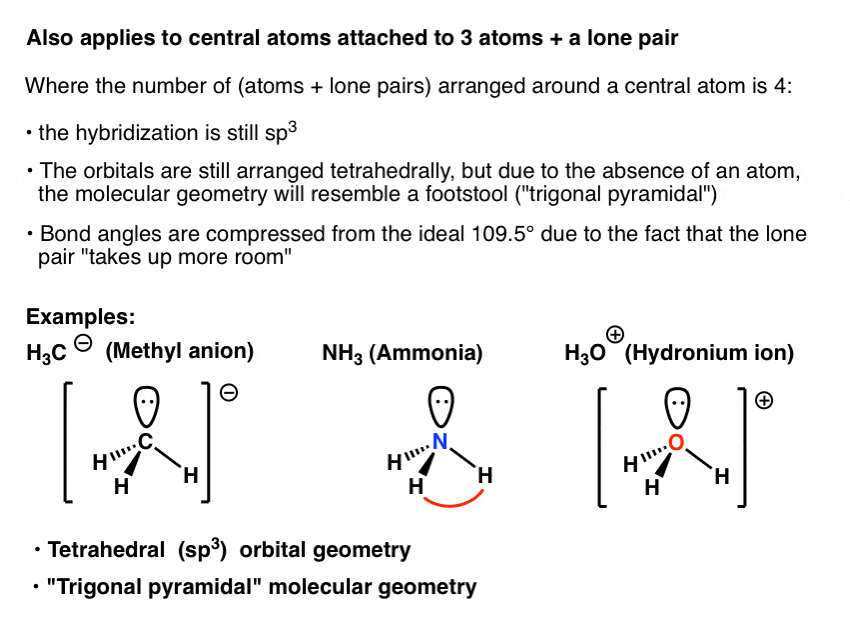
14. (a) What is the hybridization of central atom in following? NH3 ,C2 H..
We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
What Are Hybrid Orbitals and Hybridization? Master Organic Chemistry
We'll look at how to figure out if nh 3 is. The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
SOLVED Number of bonding electron pairs (bp) Molecular Geometry
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if nh 3 is.
[ANSWERED] A. What is the hybridization of the central atom in IF5
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. We'll look at how to figure out if nh 3 is. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.
We'll Look At How To Figure Out If Nh 3 Is.
The hybridization of nh₃ (ammonia) involves the combination of one nitrogen (n) atom's 2s orbital and three 2p orbitals to form four equivalent sp³. Ammonia (nh 3) is sp 3 hybridized, or to be more specific, the central atom of ammonia, nitrogen.





![[ANSWERED] A. What is the hybridization of the central atom in IF5](https://media.kunduz.com/media/sug-question/raw/52262186-1659250506.6599846.jpeg?h=512)