What Is The Electron Configuration Of Br
What Is The Electron Configuration Of Br - If it shows unpaired electrons, then the substance. Elements are organised into blocks by the orbital type in which the outer electrons are found. View rotating bohr models for all 118. Bromine belongs to group 17 which is known as halogens. These blocks are named for the characteristic spectra. Access detailed info on all elements: The magnetic form of a substance can be determined by examining its electron configuration: Atomic mass, electron configurations, charges, and more.
Atomic mass, electron configurations, charges, and more. Bromine belongs to group 17 which is known as halogens. Access detailed info on all elements: View rotating bohr models for all 118. These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. The magnetic form of a substance can be determined by examining its electron configuration: If it shows unpaired electrons, then the substance.
The magnetic form of a substance can be determined by examining its electron configuration: Atomic mass, electron configurations, charges, and more. If it shows unpaired electrons, then the substance. View rotating bohr models for all 118. These blocks are named for the characteristic spectra. Bromine belongs to group 17 which is known as halogens. Access detailed info on all elements: Elements are organised into blocks by the orbital type in which the outer electrons are found.
Electron Configuration Magnesium
Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118. The magnetic form of a substance can be determined by examining its electron configuration: These blocks are named for the characteristic spectra. Bromine belongs to group 17 which is known as halogens.
Cr2+ Ground State Electron Configuration Electron Configurations
View rotating bohr models for all 118. These blocks are named for the characteristic spectra. Bromine belongs to group 17 which is known as halogens. If it shows unpaired electrons, then the substance. Atomic mass, electron configurations, charges, and more.
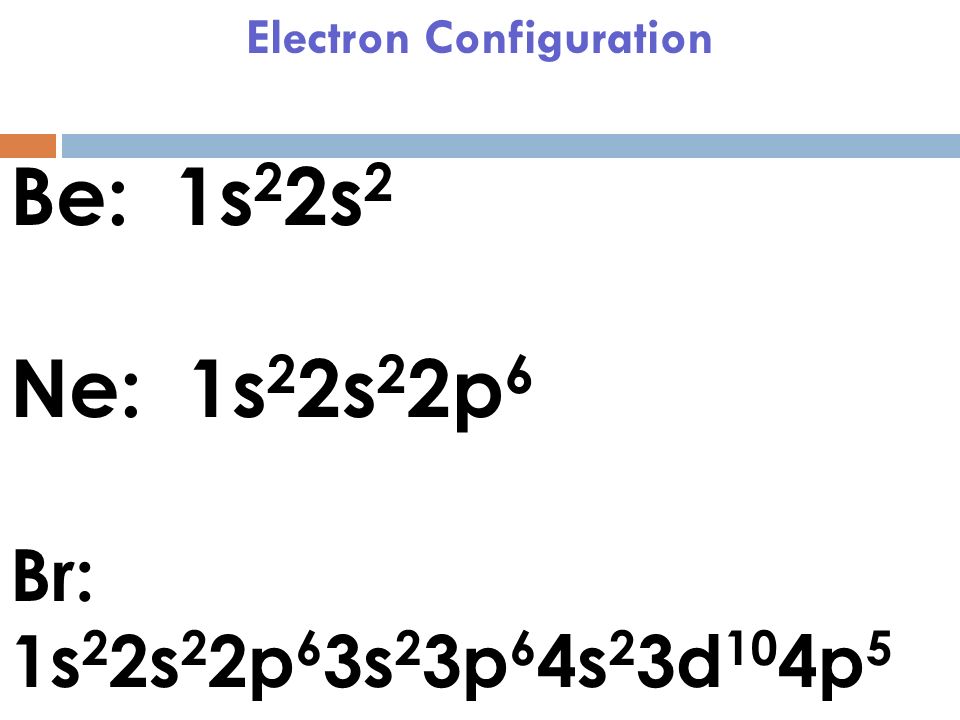
Electron Configuration For Bromine
Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine belongs to group 17 which is known as halogens. Access detailed info on all elements: If it shows unpaired electrons, then the substance. These blocks are named for the characteristic spectra.
Electron Configuration For Bromine
Access detailed info on all elements: Elements are organised into blocks by the orbital type in which the outer electrons are found. If it shows unpaired electrons, then the substance. Bromine belongs to group 17 which is known as halogens. View rotating bohr models for all 118.
Electronic Configurations Intro Chemistry LibreTexts
Elements are organised into blocks by the orbital type in which the outer electrons are found. The magnetic form of a substance can be determined by examining its electron configuration: These blocks are named for the characteristic spectra. Bromine belongs to group 17 which is known as halogens. Atomic mass, electron configurations, charges, and more.
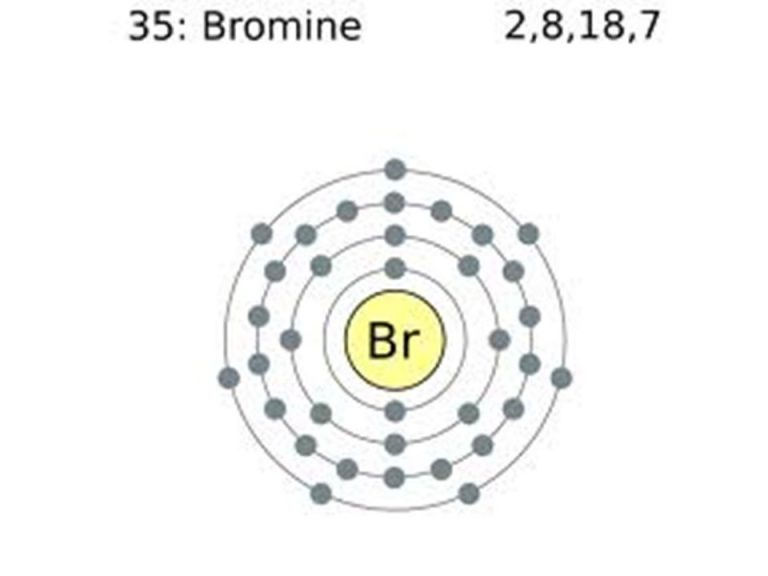
Bromine Electron Configuration (Br) with Orbital Diagram
Bromine belongs to group 17 which is known as halogens. These blocks are named for the characteristic spectra. The magnetic form of a substance can be determined by examining its electron configuration: Atomic mass, electron configurations, charges, and more. Elements are organised into blocks by the orbital type in which the outer electrons are found.
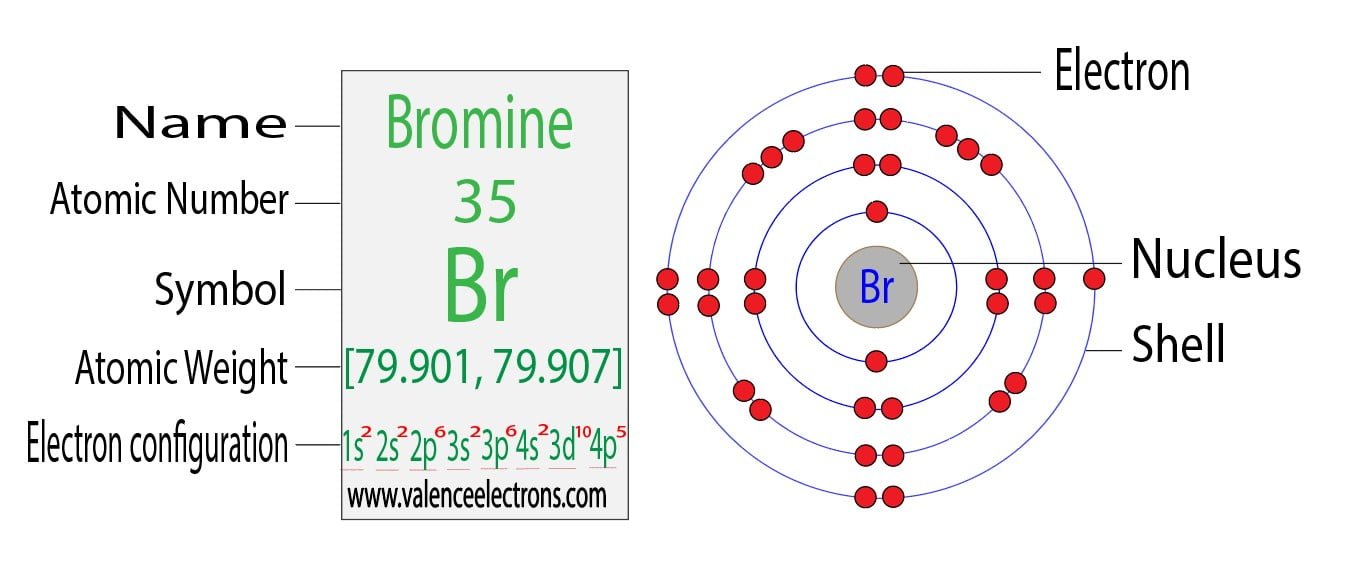
Atom Diagrams Electron Configurations of the Elements
The magnetic form of a substance can be determined by examining its electron configuration: View rotating bohr models for all 118. Bromine belongs to group 17 which is known as halogens. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements:
Bromine Electron Configuration (Br) with Orbital Diagram
View rotating bohr models for all 118. Access detailed info on all elements: Elements are organised into blocks by the orbital type in which the outer electrons are found. The magnetic form of a substance can be determined by examining its electron configuration: These blocks are named for the characteristic spectra.
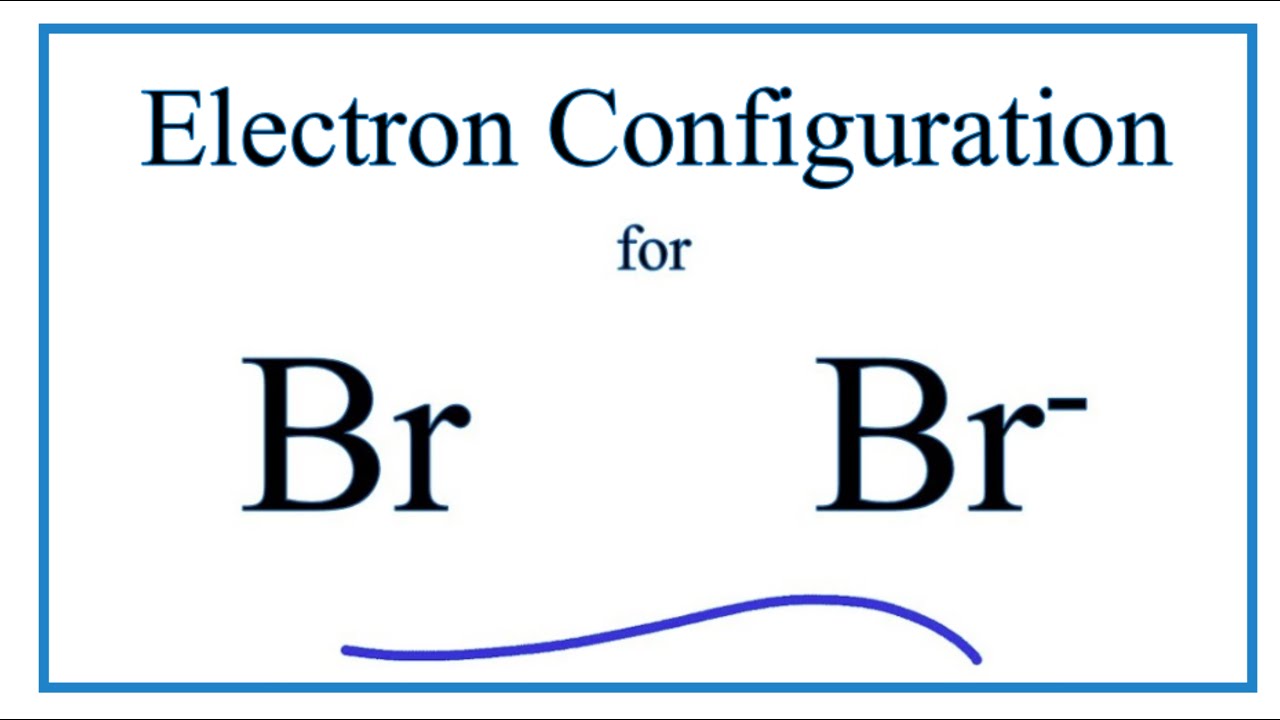
Br Electron Configuration (Bromide Ion) YouTube
The magnetic form of a substance can be determined by examining its electron configuration: Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine belongs to group 17 which is known as halogens. If it shows unpaired electrons, then the substance. Access detailed info on all elements:
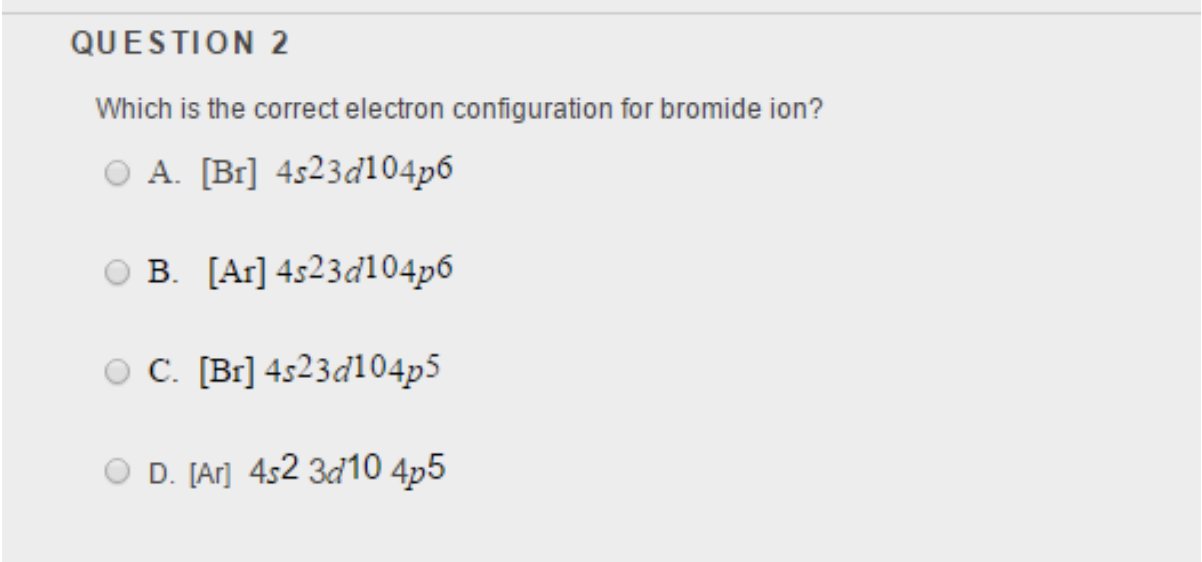
Solved QUESTION 2 Which is the correct electron
Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine belongs to group 17 which is known as halogens. These blocks are named for the characteristic spectra. Atomic mass, electron configurations, charges, and more. Access detailed info on all elements:
The Magnetic Form Of A Substance Can Be Determined By Examining Its Electron Configuration:
Elements are organised into blocks by the orbital type in which the outer electrons are found. View rotating bohr models for all 118. Atomic mass, electron configurations, charges, and more. These blocks are named for the characteristic spectra.
Bromine Belongs To Group 17 Which Is Known As Halogens.
Access detailed info on all elements: If it shows unpaired electrons, then the substance.




:max_bytes(150000):strip_icc()/Bromine-58b601f93df78cdcd83d2817.jpg)