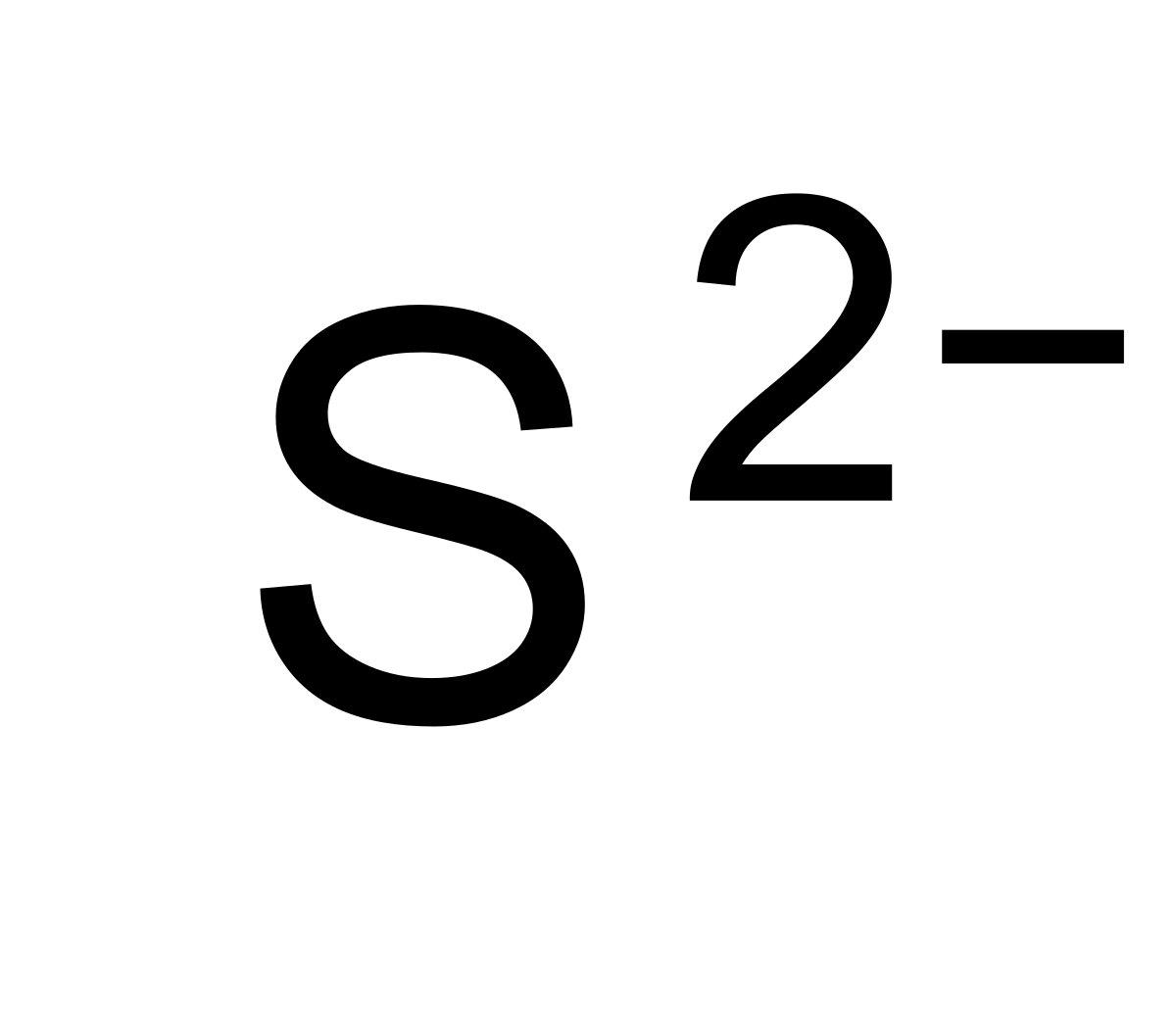What Is The Charge On A Sulfide Ion
What Is The Charge On A Sulfide Ion - Sulfide solutions develop the characteristic rotten. Post any question and get. It gains two electrons to achieve a full octet and attain a stable electronic configuration. Not the question you’re looking for? Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. To obtain an octet, sodium forms an ion with a 1+ charge, while the sulfide ion has a 2− charge. Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Here’s the best way to solve it. Two sodium 1+ ions are needed to balance the 2−.
Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis. Post any question and get. Sulfide solutions develop the characteristic rotten. Here’s the best way to solve it. Two sodium 1+ ions are needed to balance the 2−. Not the question you’re looking for? It gains two electrons to achieve a full octet and attain a stable electronic configuration. Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. To obtain an octet, sodium forms an ion with a 1+ charge, while the sulfide ion has a 2− charge. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons.
Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis. Not the question you’re looking for? It gains two electrons to achieve a full octet and attain a stable electronic configuration. Here’s the best way to solve it. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. Two sodium 1+ ions are needed to balance the 2−. Post any question and get. To obtain an octet, sodium forms an ion with a 1+ charge, while the sulfide ion has a 2− charge. Sulfide solutions develop the characteristic rotten.
(Get Answer) 2. Given That The Charge On The Sulfide Ion Is 2 (S2
To obtain an octet, sodium forms an ion with a 1+ charge, while the sulfide ion has a 2− charge. Not the question you’re looking for? Post any question and get. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Because the ammonium ion has a 1+ charge.
AIM How to write Lewis Dot Structures (Electron Dot Structures) ppt
To obtain an octet, sodium forms an ion with a 1+ charge, while the sulfide ion has a 2− charge. Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. It gains two electrons to achieve a full octet and.
How to find Protons & Electrons for the Sulfide ion (S 2) YouTube
Post any question and get. It gains two electrons to achieve a full octet and attain a stable electronic configuration. Sulfide solutions develop the characteristic rotten. Here’s the best way to solve it. Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a.
Question Video Connecting the Group Number with the Charge of an Ion
Two sodium 1+ ions are needed to balance the 2−. Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis. It gains two electrons to achieve a full octet and attain a stable electronic configuration. Not the question you’re looking for? To obtain an octet, sodium forms an ion with a 1+ charge, while.
Découvrir 28+ imagen ion sulfure formule fr.thptnganamst.edu.vn
It gains two electrons to achieve a full octet and attain a stable electronic configuration. Two sodium 1+ ions are needed to balance the 2−. Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis. Post any question and get. To obtain an octet, sodium forms an ion with a 1+ charge, while the.
sulfur Definition, Element, Symbol, Uses, & Facts Britannica
Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis. Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. It gains two electrons to achieve a full octet and attain a stable electronic.
Sulfide Wikipedia
Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Two sodium 1+ ions are needed to balance the 2−. Post any question and get. Not the question you’re looking for?
Diagram representation of the element sulfur Vector Image
Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis. Two sodium 1+ ions are needed to balance the 2−. Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. Not the question you’re.
Ionic Bonding Elements are the simplest substances There
Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. Two sodium 1+ ions are needed to balance the 2−. Sulfide solutions develop the characteristic rotten. Here’s the best way to solve it. Not the question you’re looking for?
Sulfate définition illustrée et explications
To obtain an octet, sodium forms an ion with a 1+ charge, while the sulfide ion has a 2− charge. Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. Sulfide solutions develop the characteristic rotten. Here’s the best way.
It Gains Two Electrons To Achieve A Full Octet And Attain A Stable Electronic Configuration.
To obtain an octet, sodium forms an ion with a 1+ charge, while the sulfide ion has a 2− charge. Two sodium 1+ ions are needed to balance the 2−. Because the ammonium ion has a 1+ charge and the sulfide ion has a 2− charge, we need two ammonium ions to balance the charge on a single sulfide ion. Sulfide is a strong base, so solutions of sulfide in water are basic, due to hydrolysis.
Here’s The Best Way To Solve It.
Sulfide solutions develop the characteristic rotten. Post any question and get. In your case, the sulfide anion, s2−, carries a (2 −) negative charge, which can only mean that it gained electrons. Not the question you’re looking for?