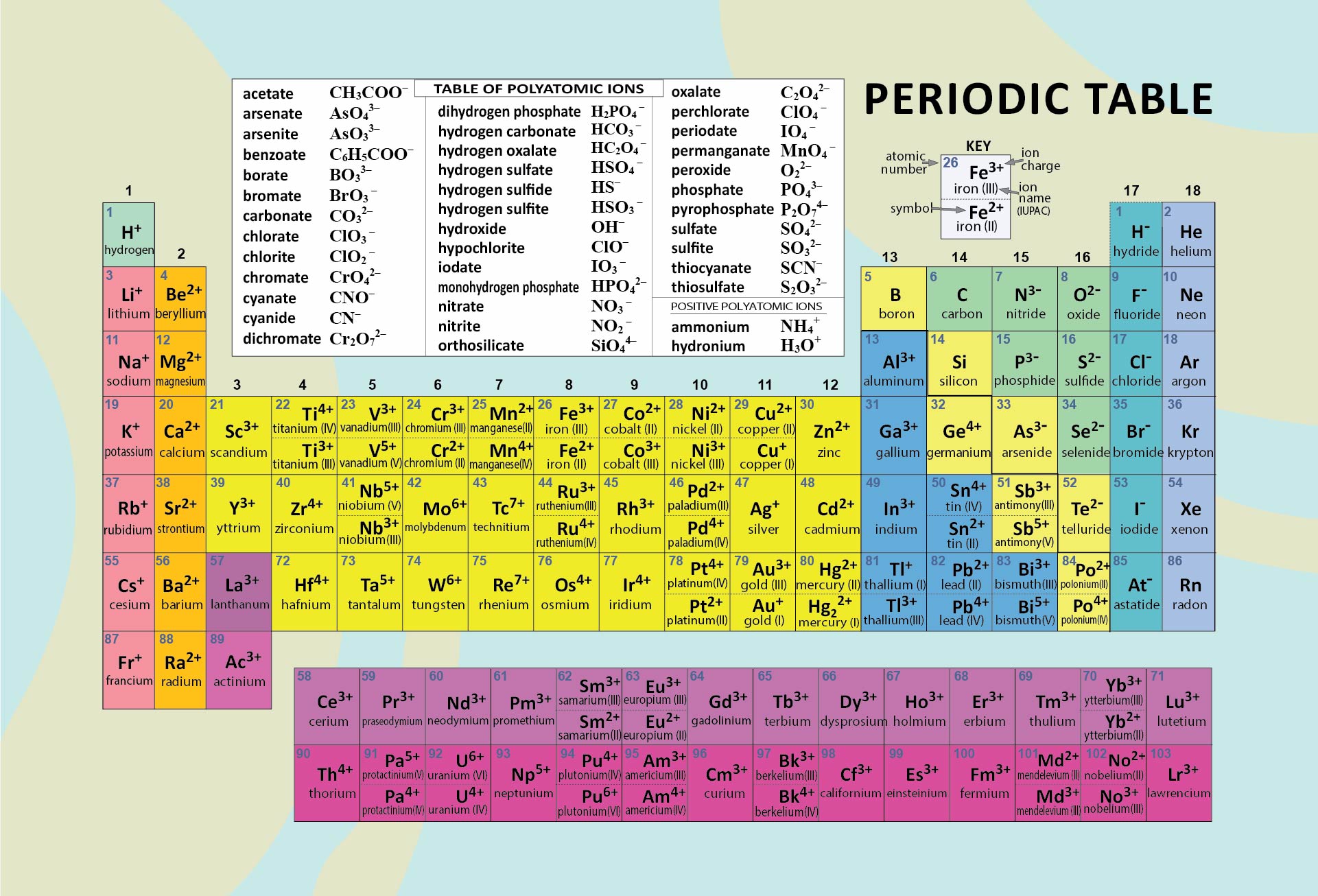
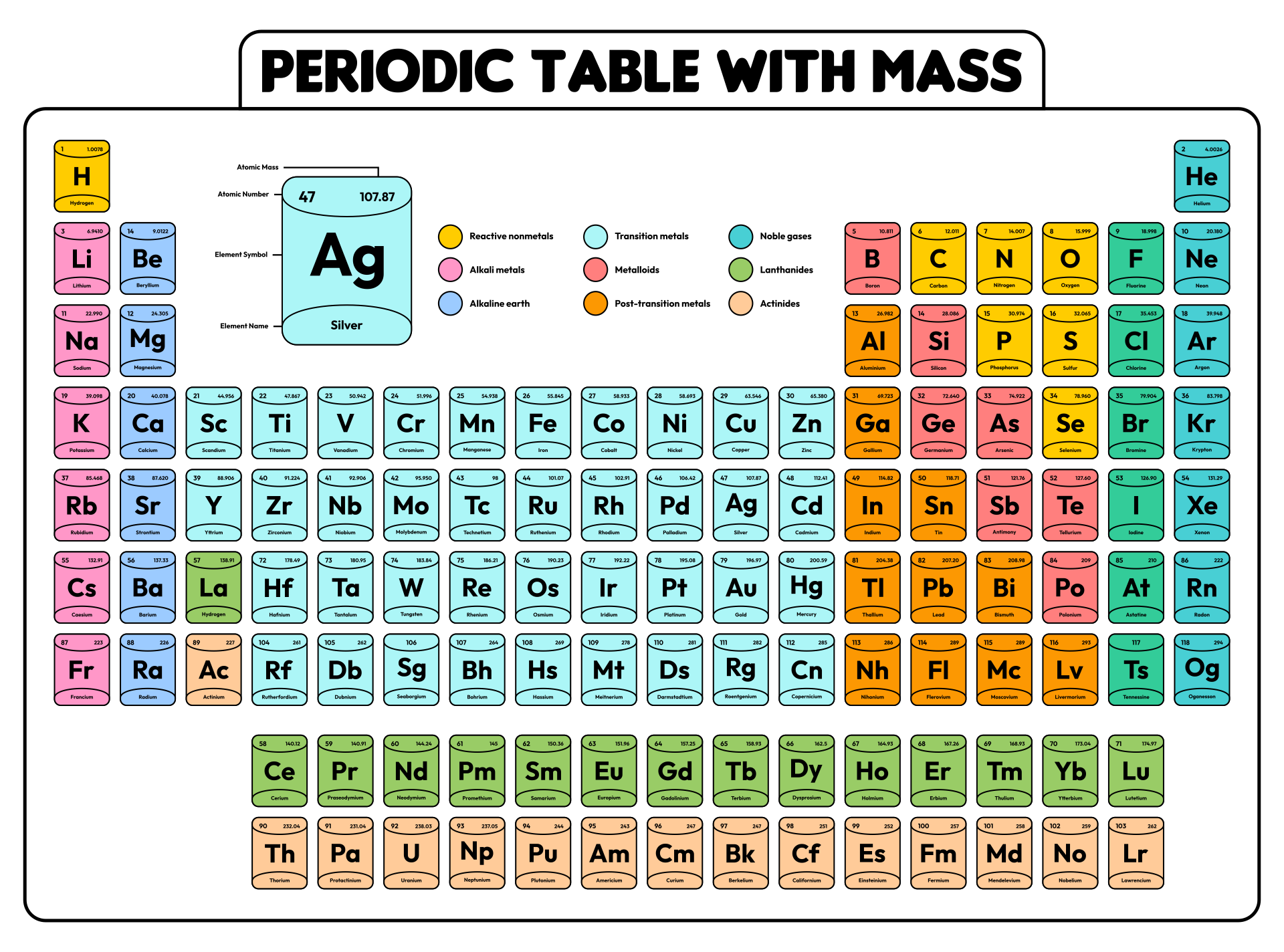
Periodic Table With Charges
Periodic Table With Charges - Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. These elements form cations with varying extents of charge. A period is the collection of elements that are arranged in a. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Atoms gain or lose valence electrons to become more stable.
The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. A period is the collection of elements that are arranged in a. These elements form cations with varying extents of charge. Atoms gain or lose valence electrons to become more stable. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are.
Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Atoms gain or lose valence electrons to become more stable. A period is the collection of elements that are arranged in a. These elements form cations with varying extents of charge.
Downloadable Periodic Table Element Charges
Atoms gain or lose valence electrons to become more stable. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. These elements form cations with varying extents of charge. Charges of +2.
Periodic Table with Charges 118 Elements
Atoms gain or lose valence electrons to become more stable. These elements form cations with varying extents of charge. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. A period is the collection of elements that.
Printable Periodic Table Element Charges
These elements form cations with varying extents of charge. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. The periodic table is a tabular arrangement of the chemical elements in the increasing order of.
Downloadable Periodic Table Element Charges
Atoms gain or lose valence electrons to become more stable. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. A period is the collection of elements that are arranged in a. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Atoms are neutral, so i'll.
Periodic Table Labeled With Charges Periodic Table Timeline
Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. A period is the collection of elements that are arranged in a. Atoms gain or lose valence electrons to become more stable. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. These elements form cations with.
Free Printable Periodic Table with Charges of Elements [PDF]
Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. These elements form cations with varying extents of charge. A period is the collection of elements that are arranged in a. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Atoms gain or lose.
Periodic Table Of Elements 10 Free PDF Printables Printablee
Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. A period is the collection of elements that are arranged in a. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Atoms gain or lose valence electrons to become more stable. These elements form.
Periodic Table With Names Of Elements And Charges
Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. These elements form cations with varying extents of charge. A period is the collection of elements that are arranged in a. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Atoms are neutral, so i'll assume.
Printable Periodic Table with Charges 2015
Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. These elements form cations with varying extents of charge. Atoms gain or lose valence electrons to become more stable. A period is the collection of.
Periodic Table with Charges PDF for Printing
Atoms gain or lose valence electrons to become more stable. These elements form cations with varying extents of charge. A period is the collection of elements that are arranged in a. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose.
A Period Is The Collection Of Elements That Are Arranged In A.
Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Atoms gain or lose valence electrons to become more stable. These elements form cations with varying extents of charge.




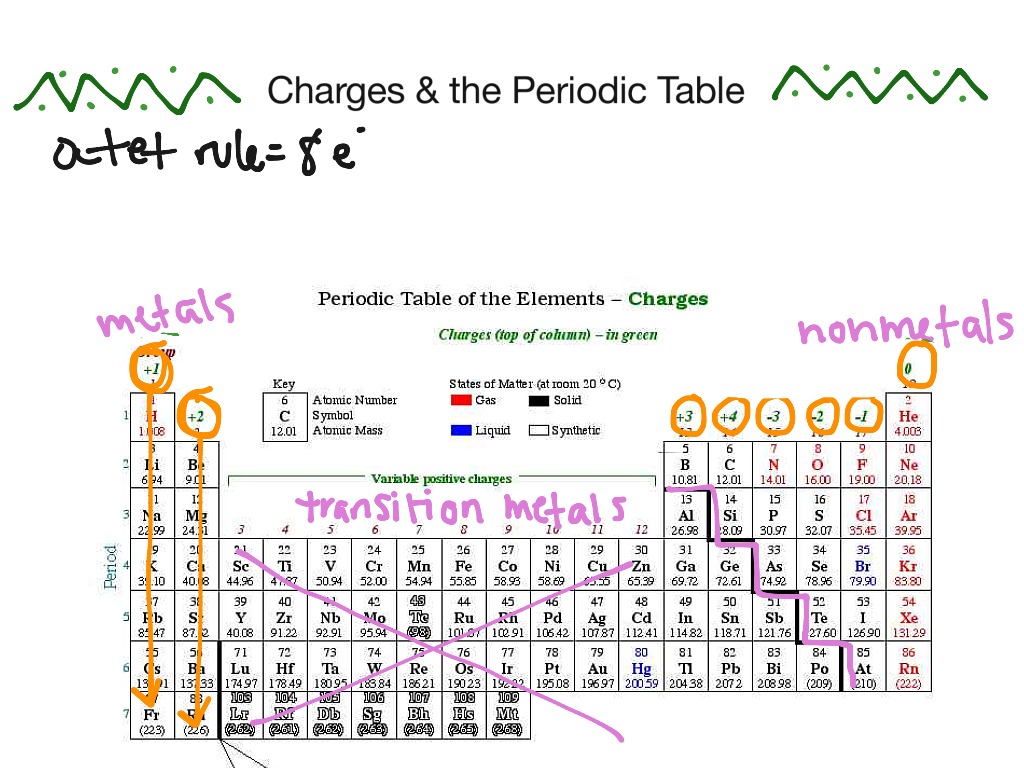
![Free Printable Periodic Table with Charges of Elements [PDF]](https://iperiodictable.com/wp-content/uploads/2020/12/Periodic-Table-with-Charges-1536x864.png)