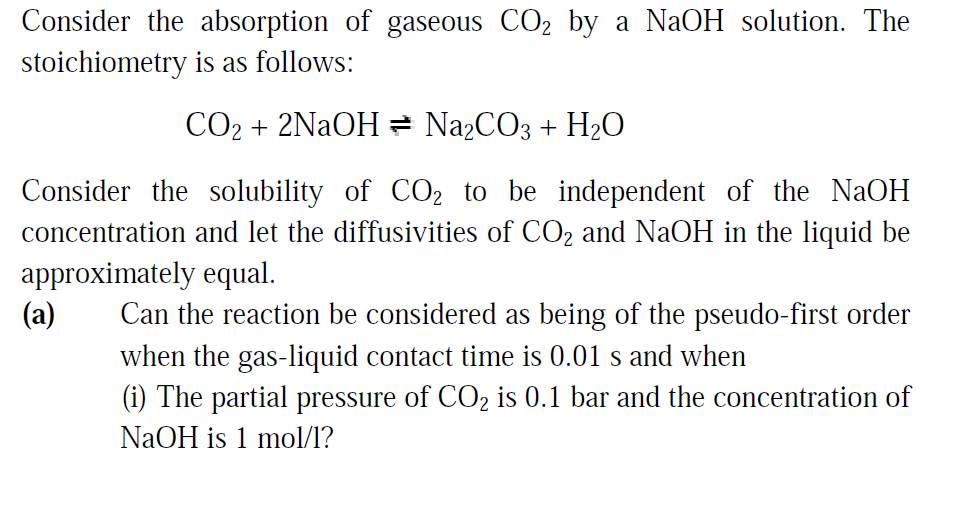Naoh Co2
Naoh Co2 - (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Sodium bicarbonate = sodium hydroxide + carbon dioxide. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Balancing of the reaction by hit and trial.
Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Balancing of the reaction by hit and trial. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2.
Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Balancing of the reaction by hit and trial.
CO2與NaOH溶液反應後產物判斷問題解析 每日頭條
1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis).
Consider the absorption of gaseous C02 by a NaOH
Sodium bicarbonate = sodium hydroxide + carbon dioxide. Balancing of the reaction by hit and trial. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if.
Na2CO3+H2O=NaOH+CO2 Balanced EquationSodium carbonate+Water=Sodium
Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o.
Figure 1 from Changes in CO2 Absorption Efficiency of NaOH Solution
(a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Balancing of the reaction by hit and trial. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for.
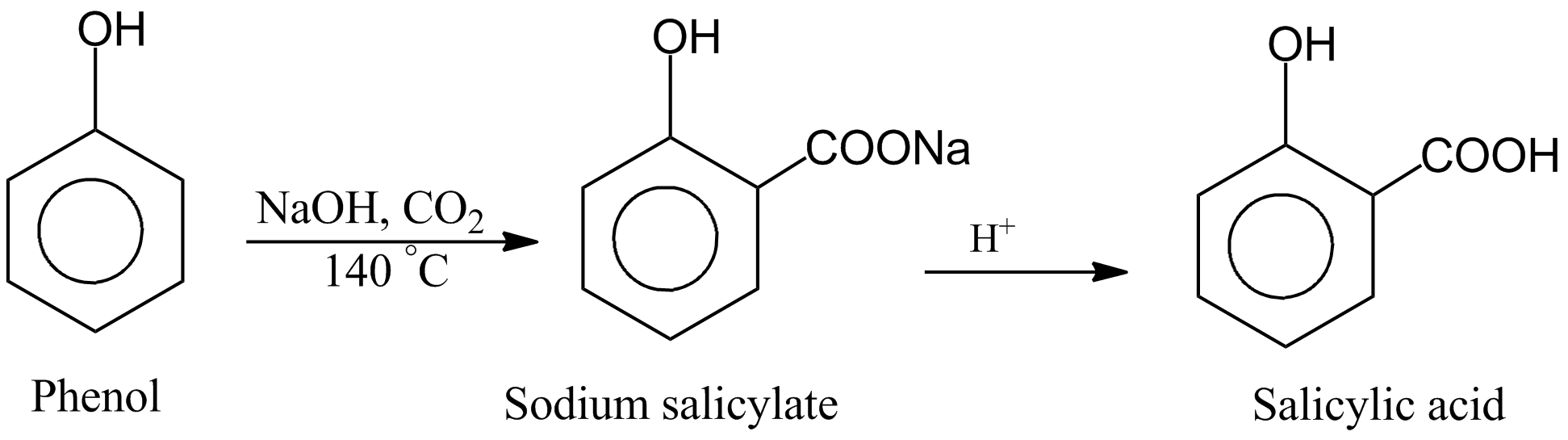
C6H6O + CO2 + NaOH 57ecrass
Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o.
Question Video Deducing the Balanced Chemical Equation for the
Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Balancing of the reaction by hit and trial. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for.
CO2 + NaOH Hấp thụ hoàn toàn 11,2 lít CO2 (đktc) vào dung dịch chứa
Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Sodium bicarbonate = sodium hydroxide.
NaOH+CO2=Na2CO3+H2O. balance the chemical equation mydocumentary838
Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Sodium bicarbonate = sodium hydroxide + carbon dioxide. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on.
How to Write the Net Ionic Equation for NaOH + CO2 = Na2CO3 + H2O YouTube
(a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Balancing of the reaction by hit and trial. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Co 2 + naoh → na 2 co 3.
NaHCO3 = NaOH + CO2
(a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of.
Sodium Bicarbonate = Sodium Hydroxide + Carbon Dioxide.
Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation.
(A) When The Alkali ($\Ce{Naoh}$) Solution Is Very Dilute ($\Mathrm{Ph} < 8$), Carbon Dioxide Will First React With Water To Form.
Balancing of the reaction by hit and trial.