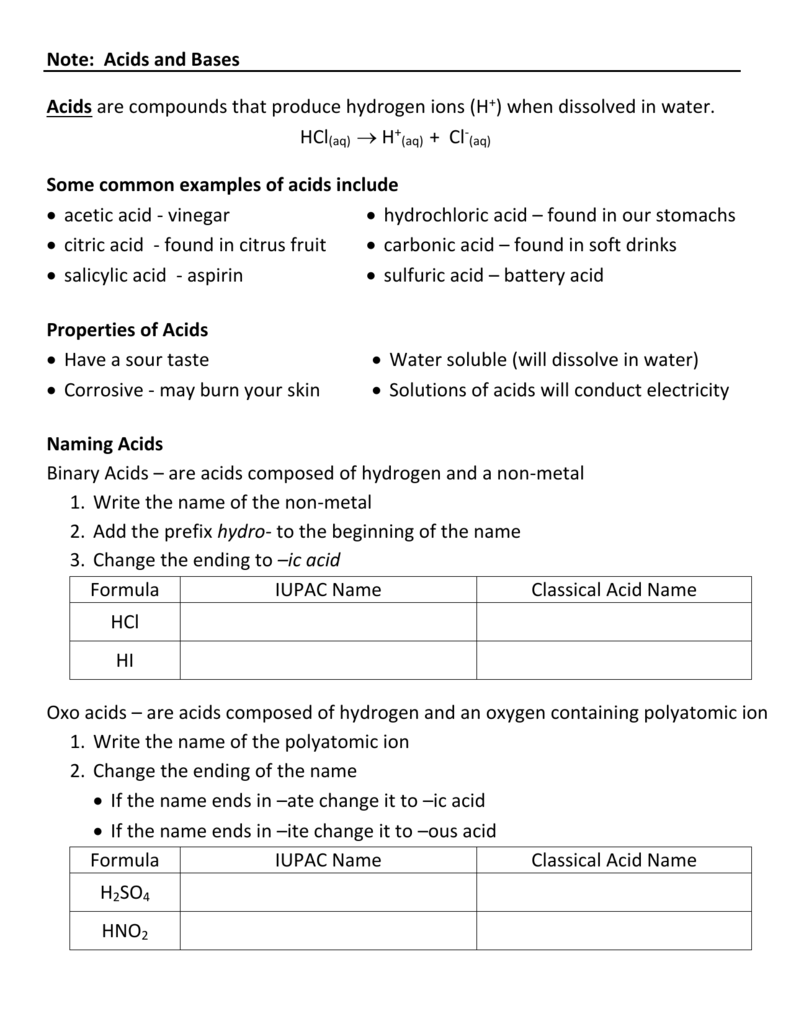
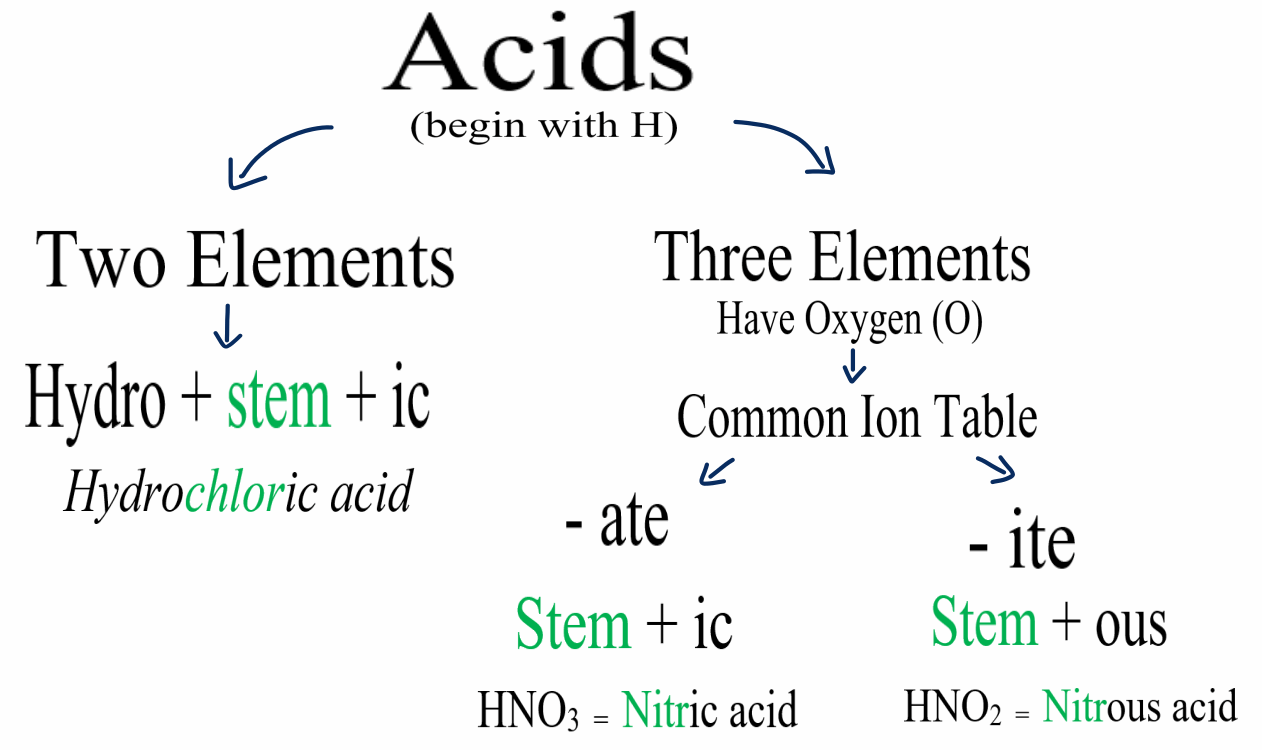
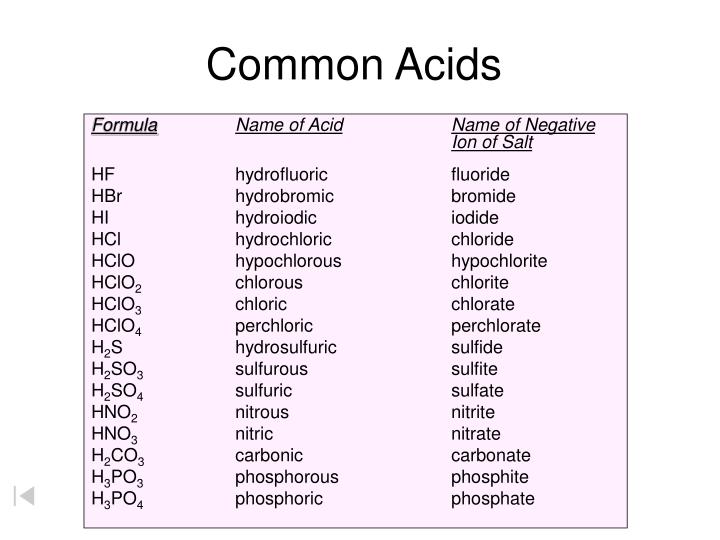
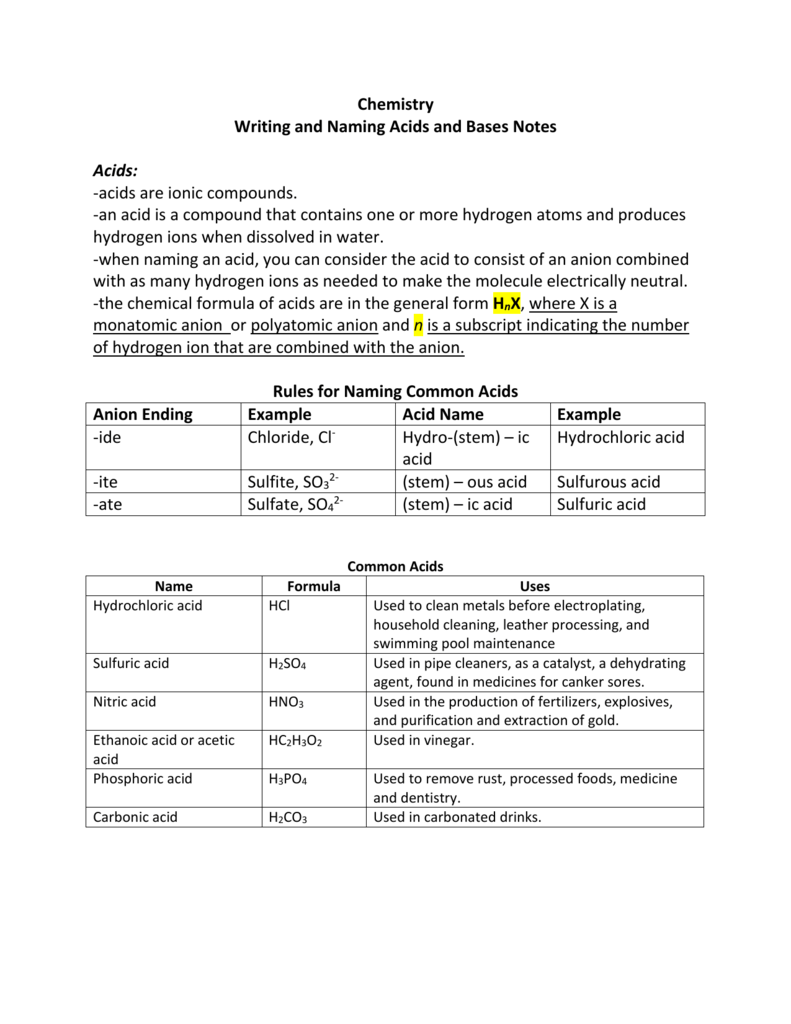
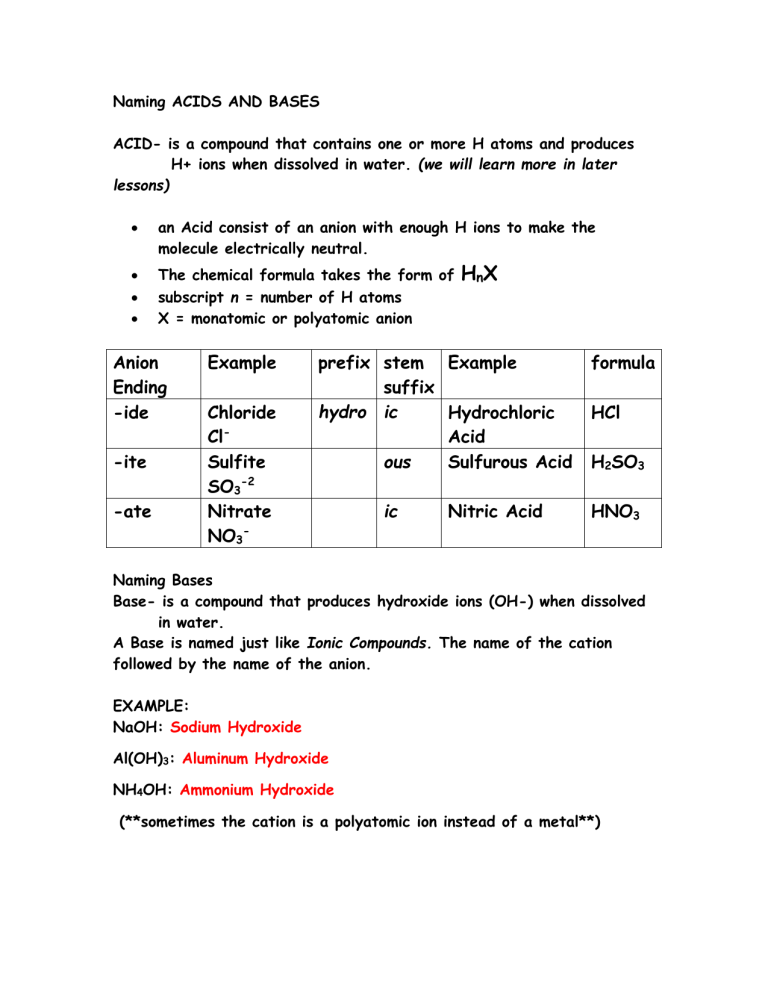
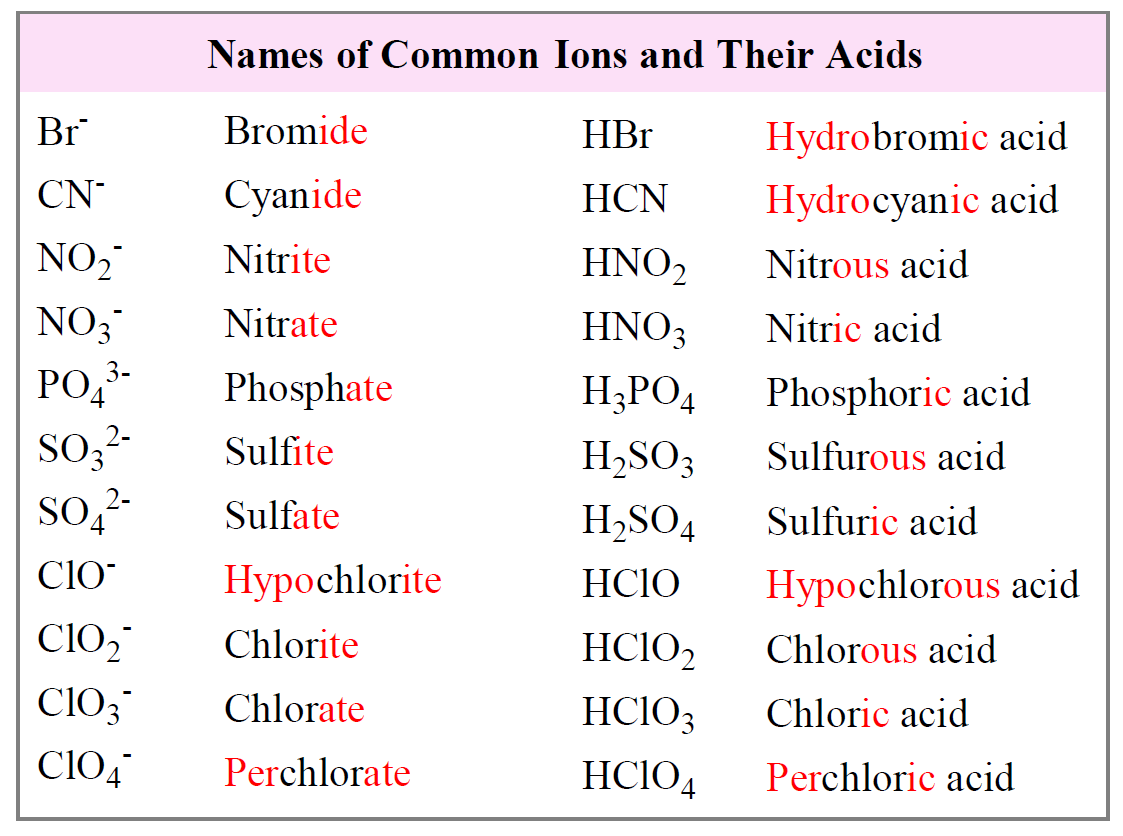
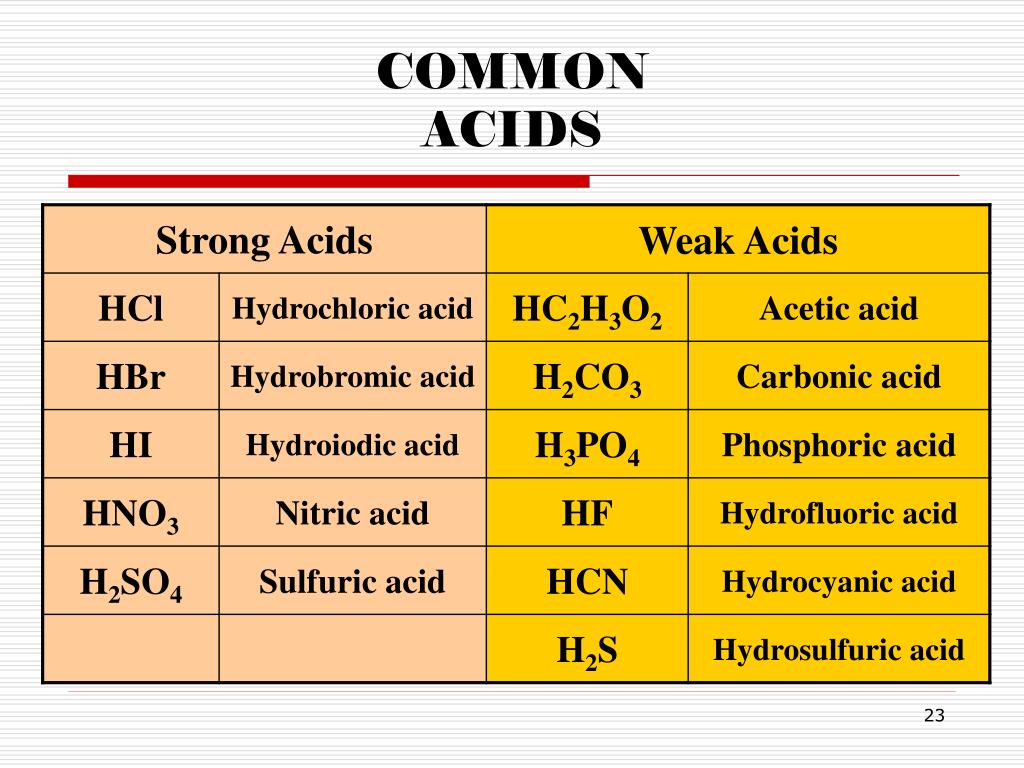
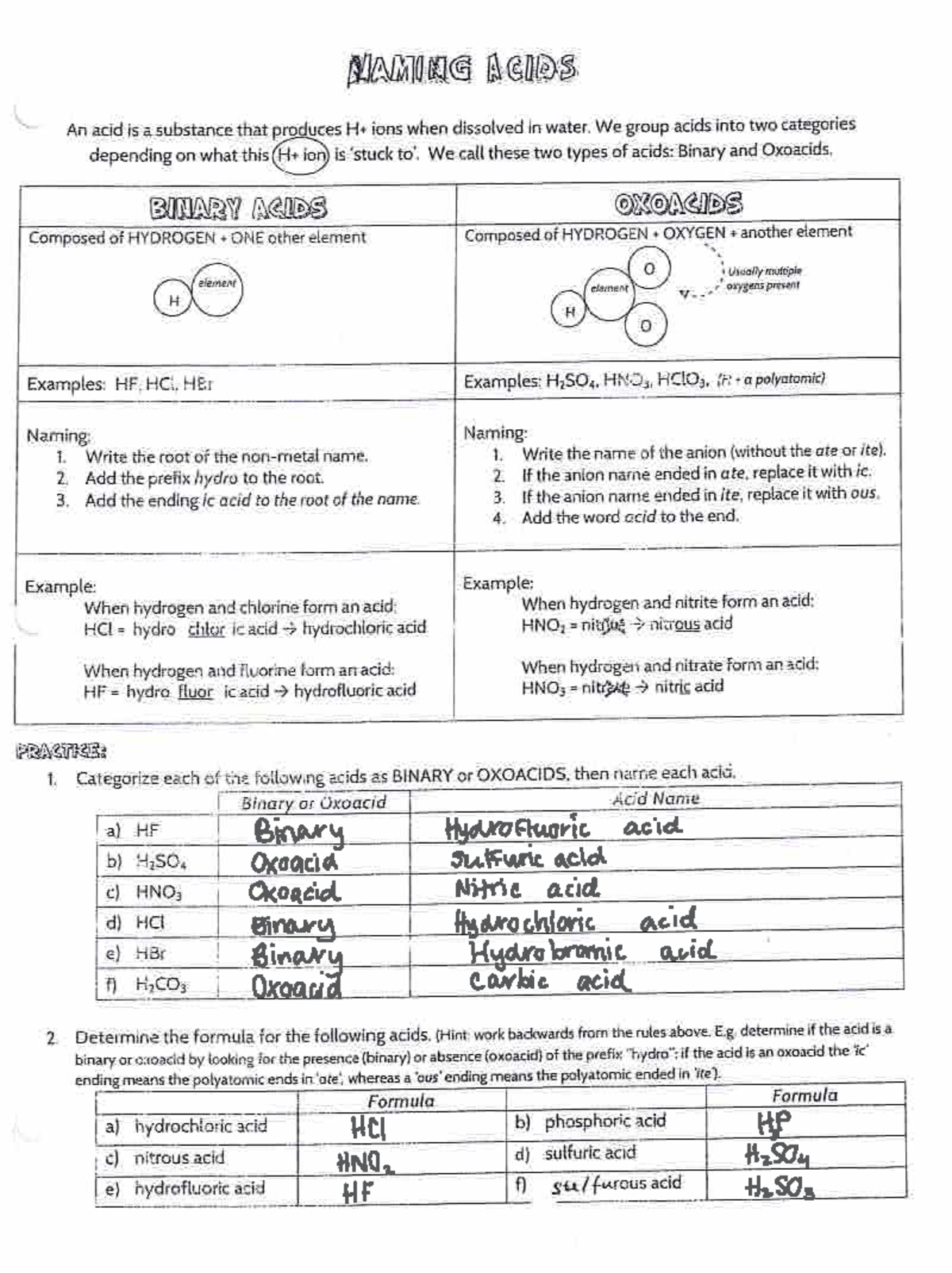
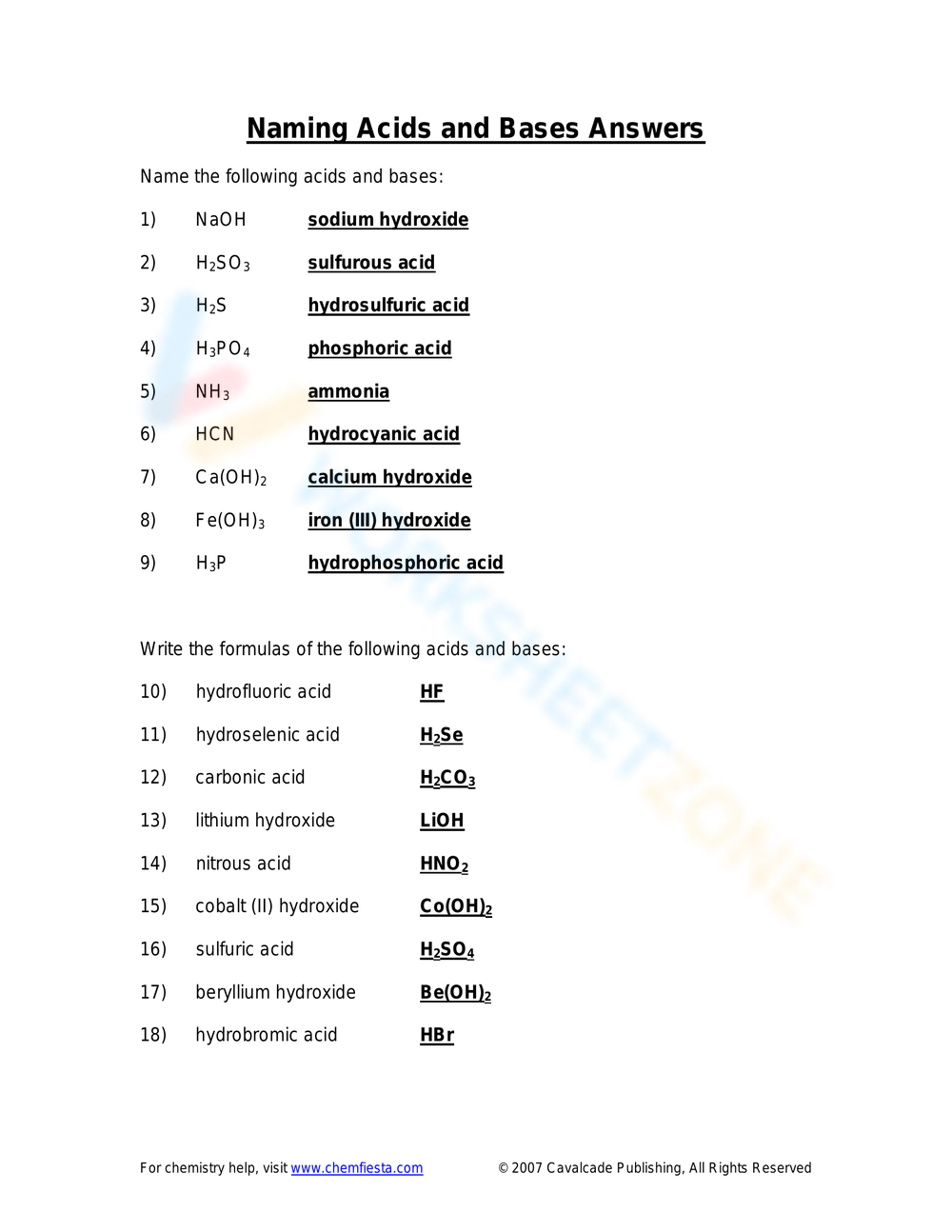
Naming Acids And Bases
Naming Acids And Bases - More practice examples on nomenclature and formulas. The general properties of acids and bases are the following. An oxyacid is an acid that consists of hydrogen, oxygen, and a. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. Bases tend to follow the same rules as ionic compounds. To name a base, add the word hydroxide to the name of the cation: In simple binary acids, one ion is attached to hydrogen.
When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. In simple binary acids, one ion is attached to hydrogen. The general properties of acids and bases are the following. Bases tend to follow the same rules as ionic compounds. More practice examples on nomenclature and formulas. An oxyacid is an acid that consists of hydrogen, oxygen, and a. To name a base, add the word hydroxide to the name of the cation:
In simple binary acids, one ion is attached to hydrogen. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. More practice examples on nomenclature and formulas. The general properties of acids and bases are the following. Bases tend to follow the same rules as ionic compounds. To name a base, add the word hydroxide to the name of the cation: An oxyacid is an acid that consists of hydrogen, oxygen, and a.
Naming Acids and Bases
Bases tend to follow the same rules as ionic compounds. An oxyacid is an acid that consists of hydrogen, oxygen, and a. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. In simple binary acids, one ion is attached to hydrogen. More practice examples on nomenclature and formulas.
Naming Acids & Bases
An oxyacid is an acid that consists of hydrogen, oxygen, and a. In simple binary acids, one ion is attached to hydrogen. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. More practice examples on nomenclature and formulas. Bases tend to follow the same rules as ionic compounds.
Common Acids And Bases You Should Know
In simple binary acids, one ion is attached to hydrogen. The general properties of acids and bases are the following. An oxyacid is an acid that consists of hydrogen, oxygen, and a. More practice examples on nomenclature and formulas. To name a base, add the word hydroxide to the name of the cation:
WritingandNamingAcidsandBasesNotes
More practice examples on nomenclature and formulas. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. The general properties of acids and bases are the following. Bases tend to follow the same rules as ionic compounds. To name a base, add the word hydroxide to the name of the cation:
Naming Acids and Bases
When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. Bases tend to follow the same rules as ionic compounds. More practice examples on nomenclature and formulas. The general properties of acids and bases are the following. In simple binary acids, one ion is attached to hydrogen.
Naming Acids and Bases Chemistry Steps
When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. In simple binary acids, one ion is attached to hydrogen. The general properties of acids and bases are the following. An oxyacid is an acid that consists of hydrogen, oxygen, and a. Bases tend to follow the same rules as ionic compounds.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
The general properties of acids and bases are the following. Bases tend to follow the same rules as ionic compounds. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. More practice examples on nomenclature and formulas. To name a base, add the word hydroxide to the name of the cation:
Naming Acids wefwefwef Studocu
An oxyacid is an acid that consists of hydrogen, oxygen, and a. The general properties of acids and bases are the following. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. Bases tend to follow the same rules as ionic compounds. To name a base, add the word hydroxide to the name.
Naming Acids And Bases Practice
The general properties of acids and bases are the following. Bases tend to follow the same rules as ionic compounds. To name a base, add the word hydroxide to the name of the cation: More practice examples on nomenclature and formulas. An oxyacid is an acid that consists of hydrogen, oxygen, and a.
Naming Acids Worksheet
More practice examples on nomenclature and formulas. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. To name a base, add the word hydroxide to the name of the cation: Bases tend to follow the same rules as ionic compounds. An oxyacid is an acid that consists of hydrogen, oxygen, and a.
When Naming Acids And Bases, Remember That An Acid Always Contains A Hydrogen Atom And An Ion.
In simple binary acids, one ion is attached to hydrogen. To name a base, add the word hydroxide to the name of the cation: An oxyacid is an acid that consists of hydrogen, oxygen, and a. More practice examples on nomenclature and formulas.
The General Properties Of Acids And Bases Are The Following.
Bases tend to follow the same rules as ionic compounds.