Hybrid Orbitals Overlap To Form
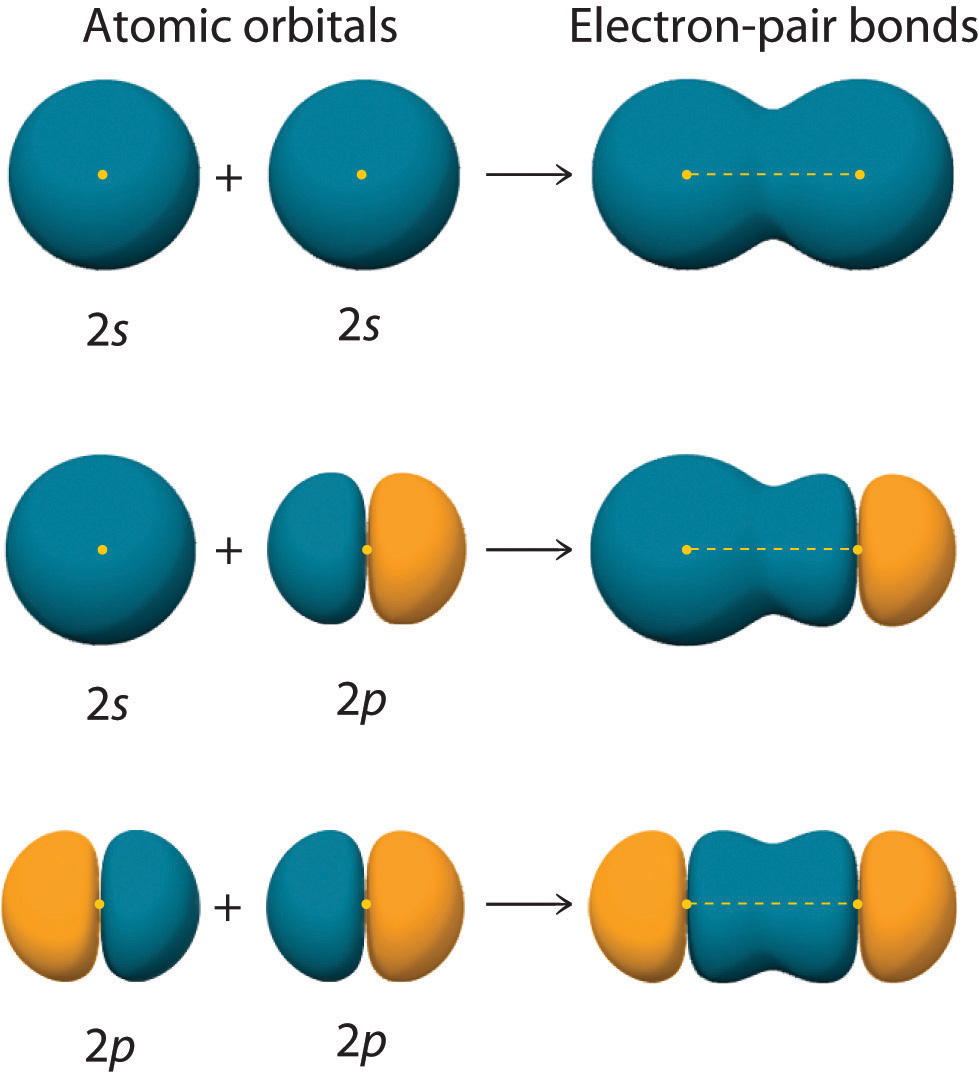
Hybrid Orbitals Overlap To Form - The type of hybrid orbitals formed in a bonded atom depends on. Unhybridized orbitals overlap to form π bonds. Hybrid orbitals overlap to form σ bonds. In the following sections, we shall discuss the. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Hybrid orbitals overlap to form σ bonds. In the following sections, we shall discuss the. Unhybridized orbitals overlap to form π bonds. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. The type of hybrid orbitals formed in a bonded atom depends on.
Hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. In the following sections, we shall discuss the. The type of hybrid orbitals formed in a bonded atom depends on. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
Hybrid orbitals overlap with ligand orbitals that can donate electron pai..
The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. Unhybridized orbitals overlap to form π bonds. In the following sections, we shall discuss the. The type of hybrid orbitals formed in a bonded atom depends on. Hybrid orbitals overlap to form σ bonds.
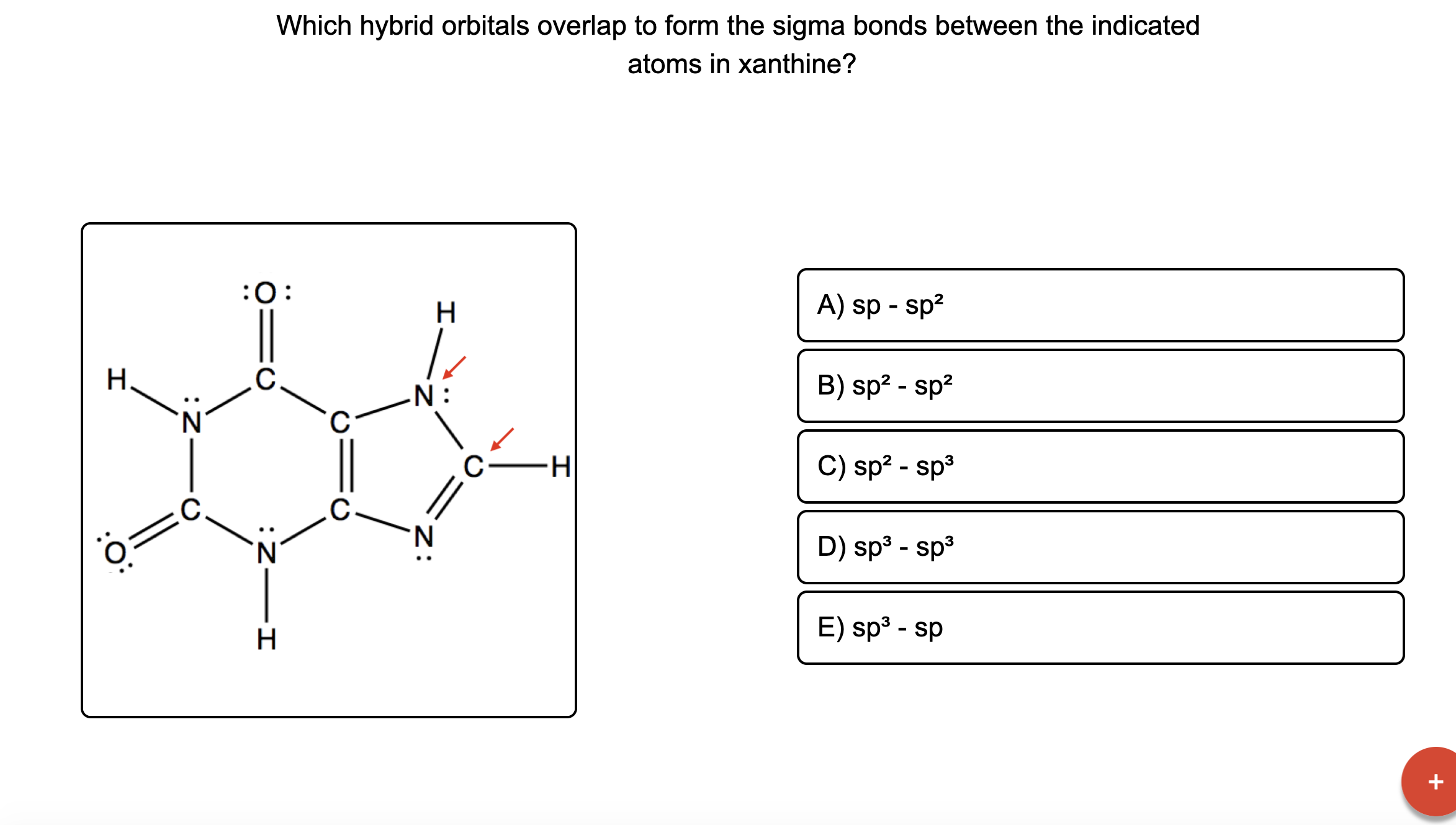
[ANSWERED] H o Z C 0 O H Which hybrid orbitals overlap to form the Kunduz
The type of hybrid orbitals formed in a bonded atom depends on. Unhybridized orbitals overlap to form π bonds. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Hybrid orbitals overlap to form σ bonds. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when.
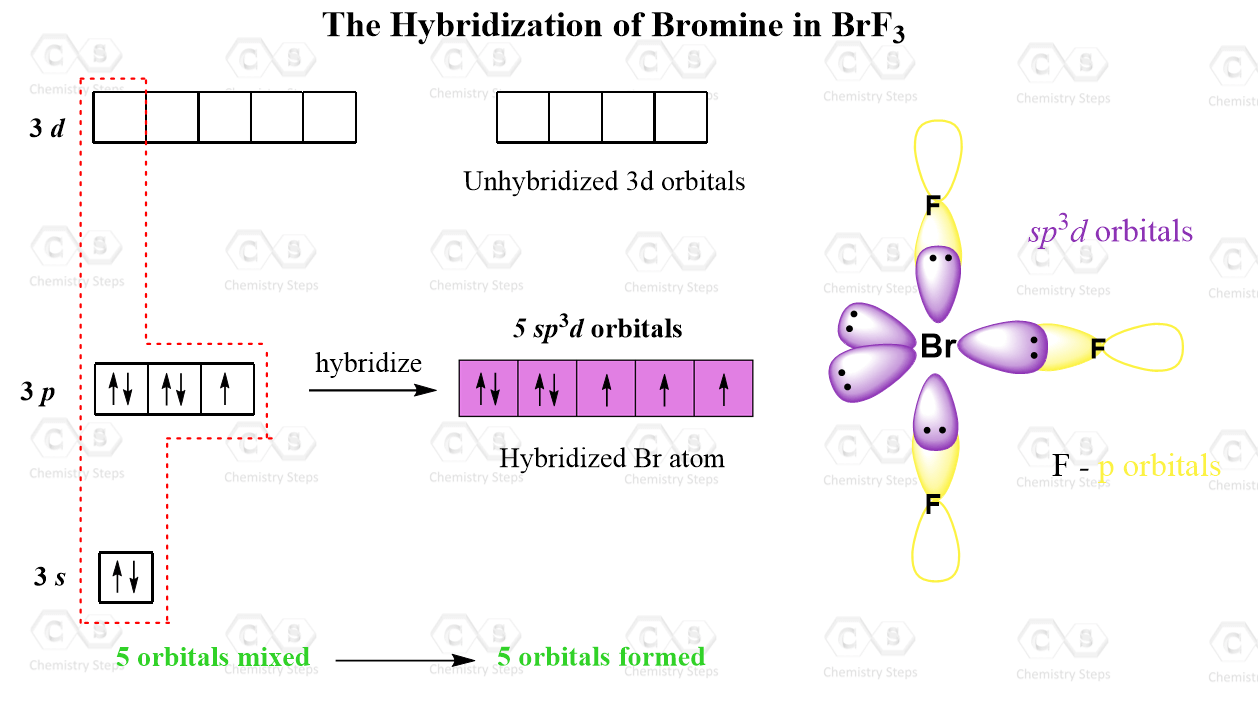
2p Orbitals
The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. Unhybridized orbitals overlap to form π bonds. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Hybrid orbitals overlap to form σ bonds. The type of hybrid orbitals formed in a bonded atom depends on.
Which hybrid orbitals overlap to form the sigma bonds between the
Hybrid orbitals overlap to form σ bonds. The type of hybrid orbitals formed in a bonded atom depends on. Unhybridized orbitals overlap to form π bonds. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
Answered Which hybrid orbitals overlap to form… bartleby
All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Hybrid orbitals overlap to form σ bonds. The type of hybrid orbitals formed in a bonded atom depends on. In the following sections, we shall discuss the. Unhybridized orbitals overlap to form π bonds.
How To Draw Hybridization Orbitals
In the following sections, we shall discuss the. The type of hybrid orbitals formed in a bonded atom depends on. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds.
Identify The Set Of Hybrid Orbitals Shown Below.? New Update
All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Unhybridized orbitals overlap to form π bonds. Hybrid orbitals overlap to form σ bonds. In the following sections, we shall discuss the. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when.
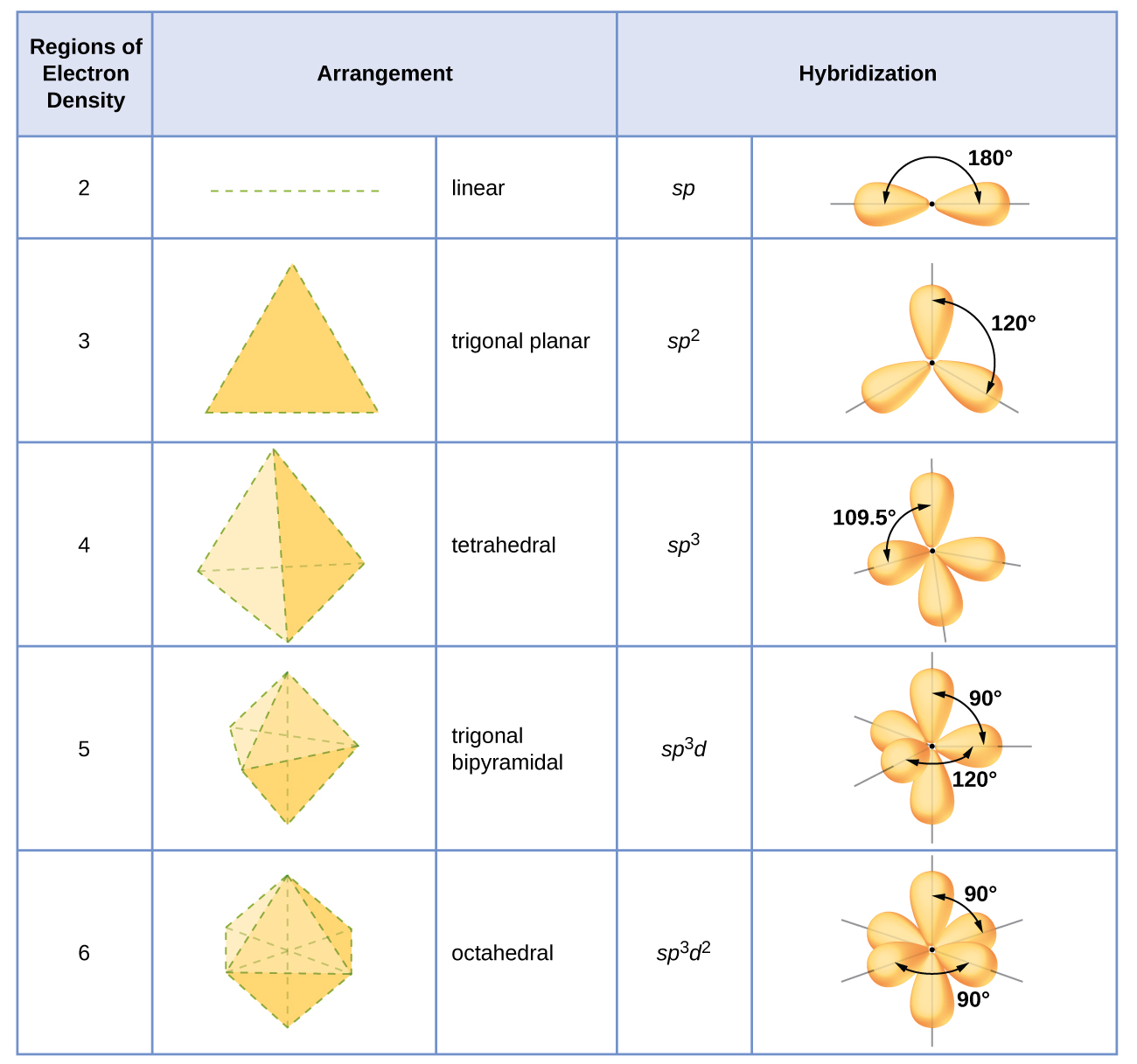
8.2 Hybrid Atomic Orbitals Chemistry LibreTexts
Hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. In the following sections, we shall discuss the. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when.
hybrid orbitals infographic. Linus Pauling's explanation of bonding
The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. Hybrid orbitals overlap to form σ bonds. In the following sections, we shall discuss the. The type of hybrid orbitals formed in a bonded atom depends on. Unhybridized orbitals overlap to form π bonds.
9.5 Hybrid Orbitals Chemistry LibreTexts
Unhybridized orbitals overlap to form π bonds. Hybrid orbitals overlap to form σ bonds. The type of hybrid orbitals formed in a bonded atom depends on. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when.
In The Following Sections, We Shall Discuss The.
The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. Hybrid orbitals overlap to form σ bonds. The type of hybrid orbitals formed in a bonded atom depends on. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.

![[ANSWERED] H o Z C 0 O H Which hybrid orbitals overlap to form the Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210518000608701743-3314603.jpg?h=512)