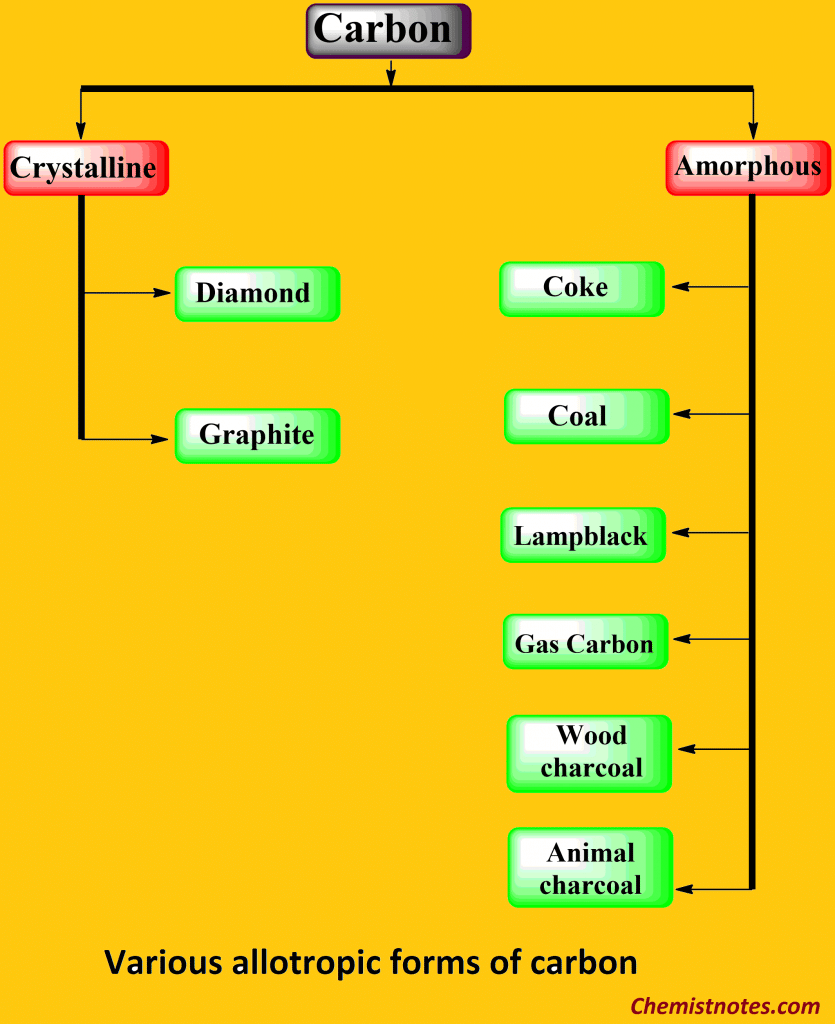
Diamond And Graphite Are Two Crystalline Forms Of Carbon
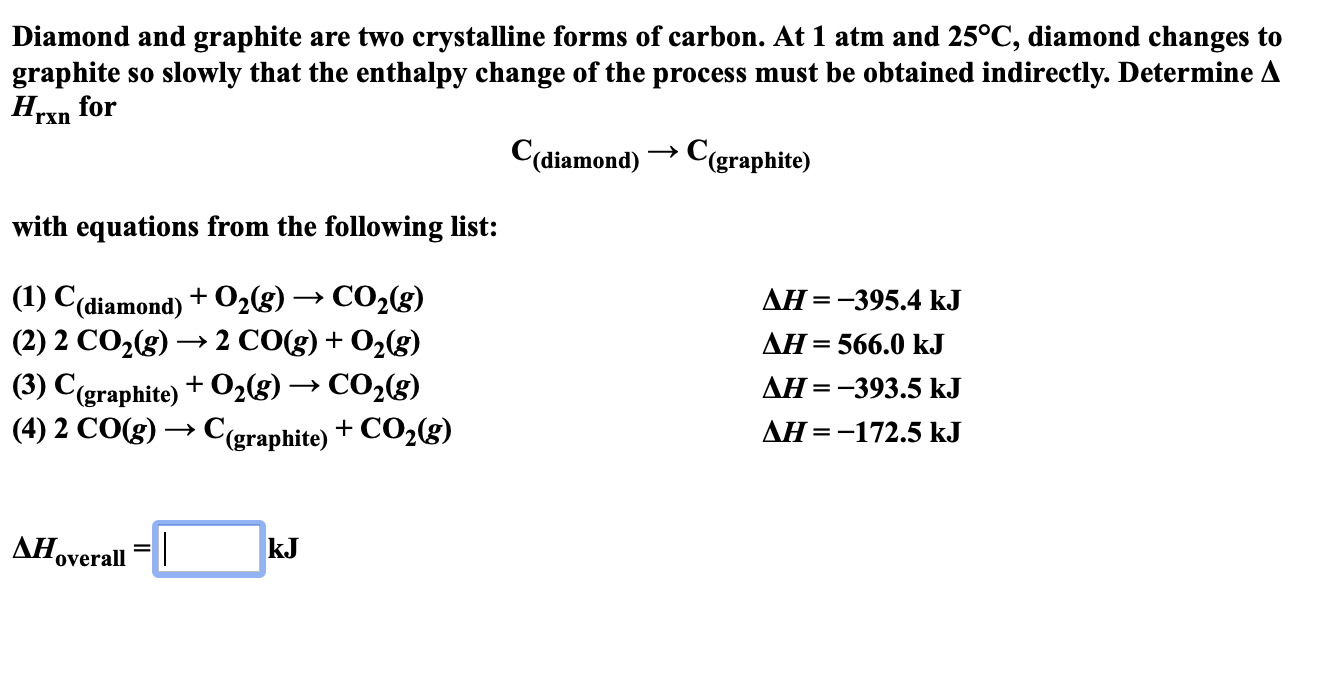
Diamond And Graphite Are Two Crystalline Forms Of Carbon - At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. Let’s learn their properties in detail.
Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Let’s learn their properties in detail. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the.
Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Let’s learn their properties in detail. Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the.
4 Properties Of Metalloids Science Trends
Diamond and graphite are two crystalline forms of carbon. Let’s learn their properties in detail. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Despite being made of the same element, their properties are. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the.
Allotropes of Carbon Introduction to Chemistry
Let’s learn their properties in detail. Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the.
Carbon graphite and diamond hires stock photography and images Alamy
Despite being made of the same element, their properties are. Diamond and graphite are two crystalline forms of carbon. Let’s learn their properties in detail. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the.
Answered Diamond and graphite are two… bartleby
Diamond and graphite are two crystalline forms of carbon. Let’s learn their properties in detail. Despite being made of the same element, their properties are. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the.
Graphite Structure PhysicsOpenLab
At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the. Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Let’s learn their properties in detail.
[Solved] ALL ABOUT CHEM. 6.48 Diamond and graphite are two crystalline
Let’s learn their properties in detail. Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
Thread by mikamckinnon "Ah, I see we're on "Crystals are Magic" day
Let’s learn their properties in detail. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the.
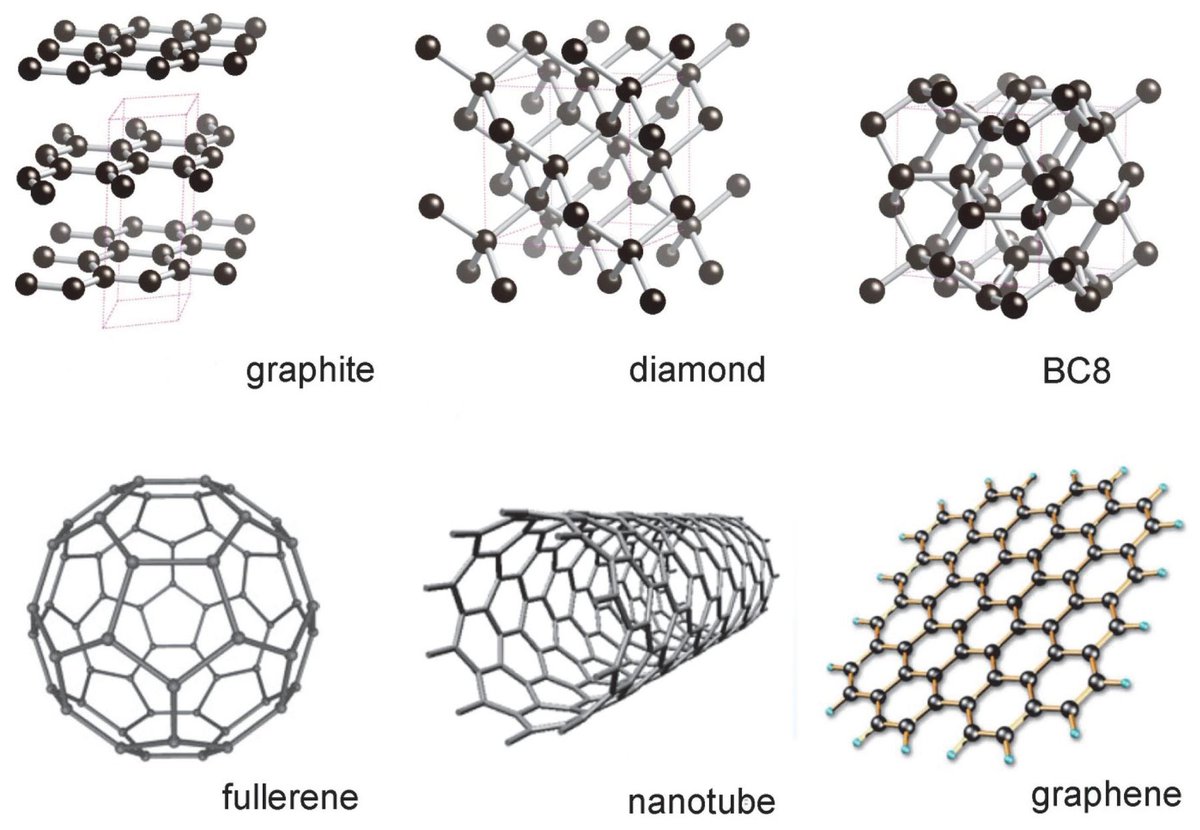
Allotropes of Carbon Structures and Important Applications
At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Diamond and graphite are two crystalline forms of carbon. Let’s learn their properties in detail. Despite being made of the same element, their properties are.
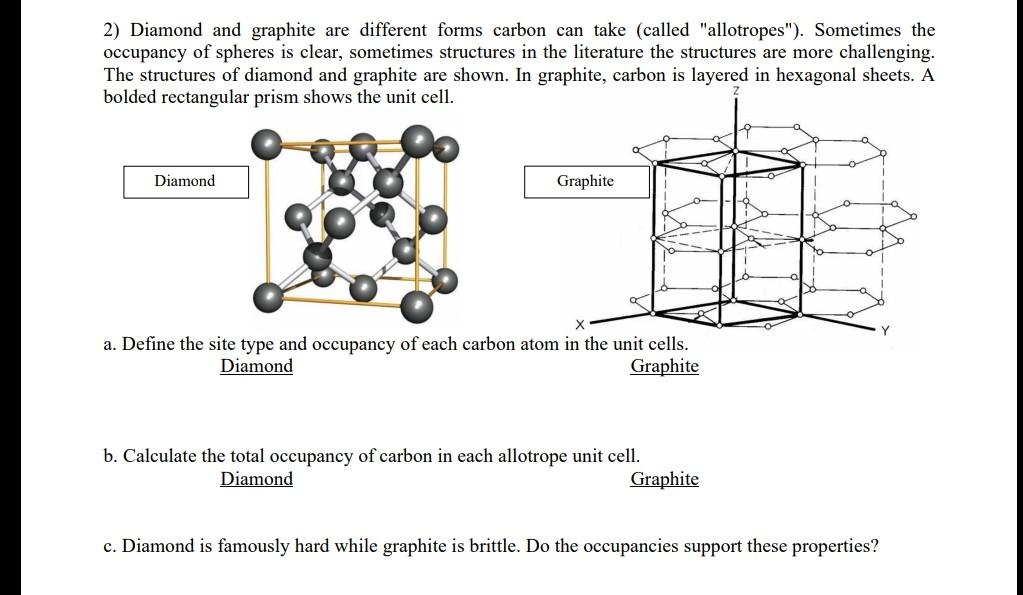
Solved 2) Diamond and graphite are different forms carbon
Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element. Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. Let’s learn their properties in detail. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the.
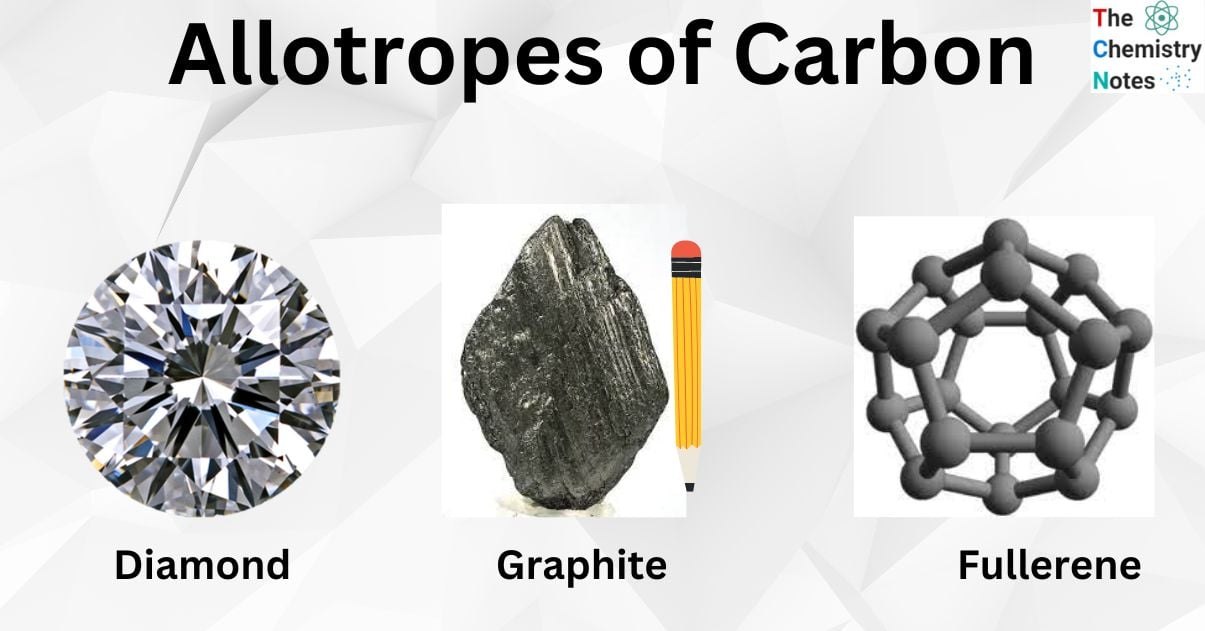
Allotropes of carbon Diamond, Graphite, and Fullerene Chemistry Notes
At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the. Let’s learn their properties in detail. Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. Diamond and graphite are two allotropes of carbon, meaning they are two different forms of the same element.
Diamond And Graphite Are Two Allotropes Of Carbon, Meaning They Are Two Different Forms Of The Same Element.
Diamond and graphite are two crystalline forms of carbon. Despite being made of the same element, their properties are. At 1 atm and 25°c, diamond changes to graphite so slowly that the enthalpy change of the. Let’s learn their properties in detail.