Aspirin Safety Data Sheet
Aspirin Safety Data Sheet - 06 jun, 2011 notice nsf reference standards are for test. General advice show this safety data sheet to the doctor in attendance. Use personal protective equipment as required. Safety data sheet version 6.12 revision date 09/07/2024 print date 09/07/2024 section 1: Inhalation may cause allergic respiratory reaction. To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade. Only select registry of toxic effects of chemical. Should not be released into the environment. Material safety data sheet name of product: Identification of the substance/mixture and of.
General advice show this safety data sheet to the doctor in attendance. Should not be released into the environment. Only select registry of toxic effects of chemical. To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade. Use personal protective equipment as required. Material safety data sheet name of product: 06 jun, 2011 notice nsf reference standards are for test. Inhalation may cause allergic respiratory reaction. Identification of the substance/mixture and of. Safety data sheet version 6.12 revision date 09/07/2024 print date 09/07/2024 section 1:
Use personal protective equipment as required. Should not be released into the environment. Safety data sheet version 6.12 revision date 09/07/2024 print date 09/07/2024 section 1: General advice show this safety data sheet to the doctor in attendance. 06 jun, 2011 notice nsf reference standards are for test. Inhalation may cause allergic respiratory reaction. Only select registry of toxic effects of chemical. Material safety data sheet name of product: Identification of the substance/mixture and of. To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade.
BUY Aspirin Regular Strength (Aspirin) 325 mg/1 from GNH India at the
General advice show this safety data sheet to the doctor in attendance. Safety data sheet version 6.12 revision date 09/07/2024 print date 09/07/2024 section 1: Identification of the substance/mixture and of. 06 jun, 2011 notice nsf reference standards are for test. Use personal protective equipment as required.
Buy Equate Adult Low Dose Aspirin Safety Coated Tablets, 81 mg, 300
Identification of the substance/mixture and of. Use personal protective equipment as required. To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade. Inhalation may cause allergic respiratory reaction. Should not be released into the environment.
Material Safety Data Sheet ?Acetaminophen? MSDS
To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade. Material safety data sheet name of product: Inhalation may cause allergic respiratory reaction. Use personal protective equipment as required. General advice show this safety data sheet to the doctor in attendance.
Pack) Equate Aspirin Pain Reliever/Fever Reducer Coated, 54 OFF
To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade. Identification of the substance/mixture and of. Should not be released into the environment. Use personal protective equipment as required. General advice show this safety data sheet to the doctor in attendance.
DailyMed ASPIRIN 81 MG aspirin tablet, coated
Should not be released into the environment. Identification of the substance/mixture and of. 06 jun, 2011 notice nsf reference standards are for test. Material safety data sheet name of product: Inhalation may cause allergic respiratory reaction.
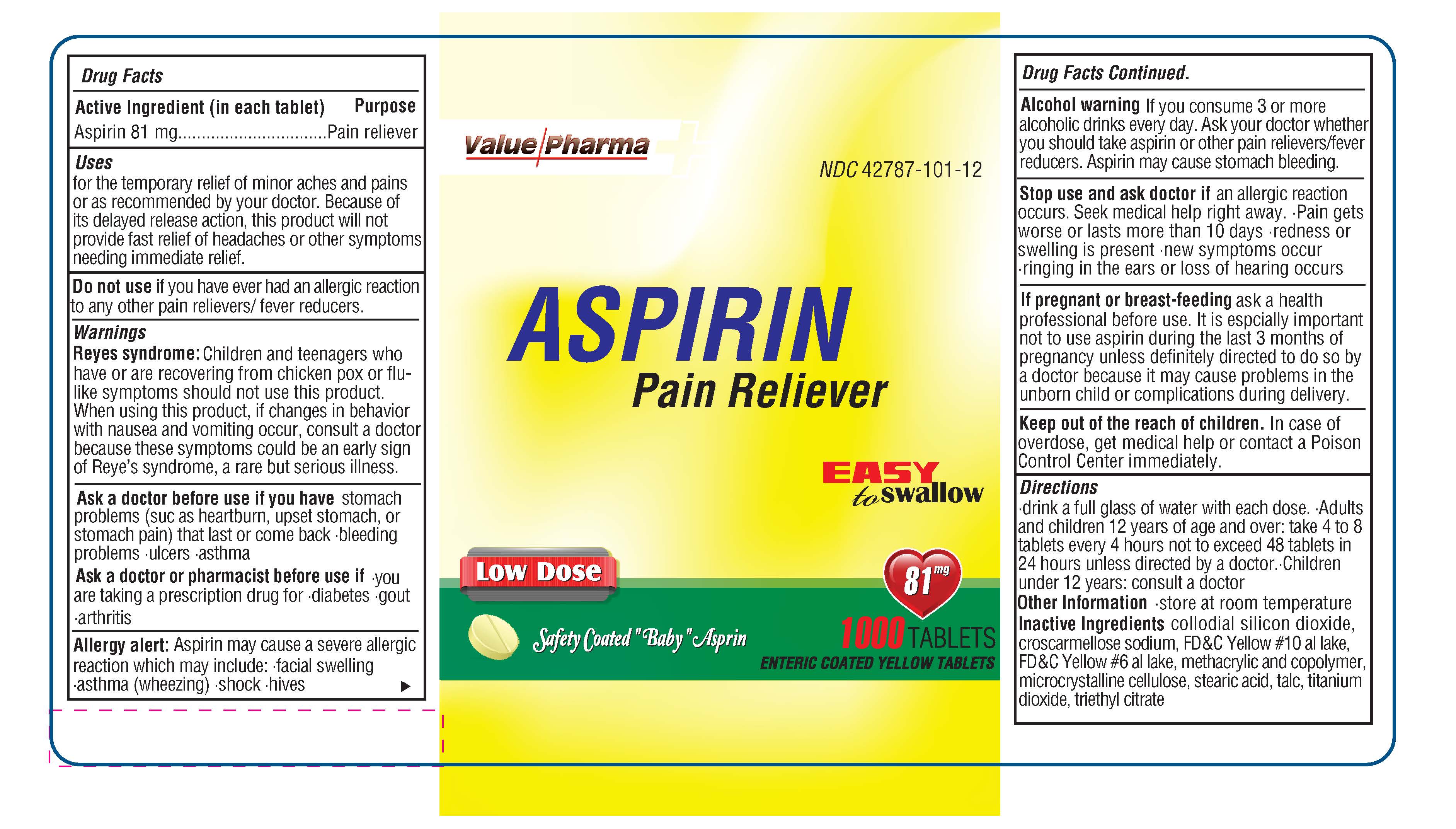
DRUG FACTS
Use personal protective equipment as required. Material safety data sheet name of product: Should not be released into the environment. 06 jun, 2011 notice nsf reference standards are for test. To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade.
Material Safety Data Sheet Acetylsalicylic acid MSDS 00300
Safety data sheet version 6.12 revision date 09/07/2024 print date 09/07/2024 section 1: Inhalation may cause allergic respiratory reaction. Use personal protective equipment as required. Only select registry of toxic effects of chemical. 06 jun, 2011 notice nsf reference standards are for test.
Print the Aspirin fact sheet DrugInfo
Should not be released into the environment. General advice show this safety data sheet to the doctor in attendance. Only select registry of toxic effects of chemical. Use personal protective equipment as required. Material safety data sheet name of product:
Equate Adult Low Dose Aspirin Safety Coated Tablets, 81 mg, 500 Count
Inhalation may cause allergic respiratory reaction. Safety data sheet version 6.12 revision date 09/07/2024 print date 09/07/2024 section 1: Only select registry of toxic effects of chemical. Identification of the substance/mixture and of. To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade.
Safety Data Sheet Aspirin Powder PDF Aspirin Toxicity
General advice show this safety data sheet to the doctor in attendance. Safety data sheet version 6.12 revision date 09/07/2024 print date 09/07/2024 section 1: Should not be released into the environment. To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade. Material safety data sheet name of product:
General Advice Show This Safety Data Sheet To The Doctor In Attendance.
To osha hcs printing date 07/18/2023 revision date 07/18/2023 57.0.2 * 1 identification · product identifier · trade. Only select registry of toxic effects of chemical. Should not be released into the environment. Material safety data sheet name of product:
06 Jun, 2011 Notice Nsf Reference Standards Are For Test.
Inhalation may cause allergic respiratory reaction. Use personal protective equipment as required. Identification of the substance/mixture and of. Safety data sheet version 6.12 revision date 09/07/2024 print date 09/07/2024 section 1: