An Ionic Bond Forms Between A
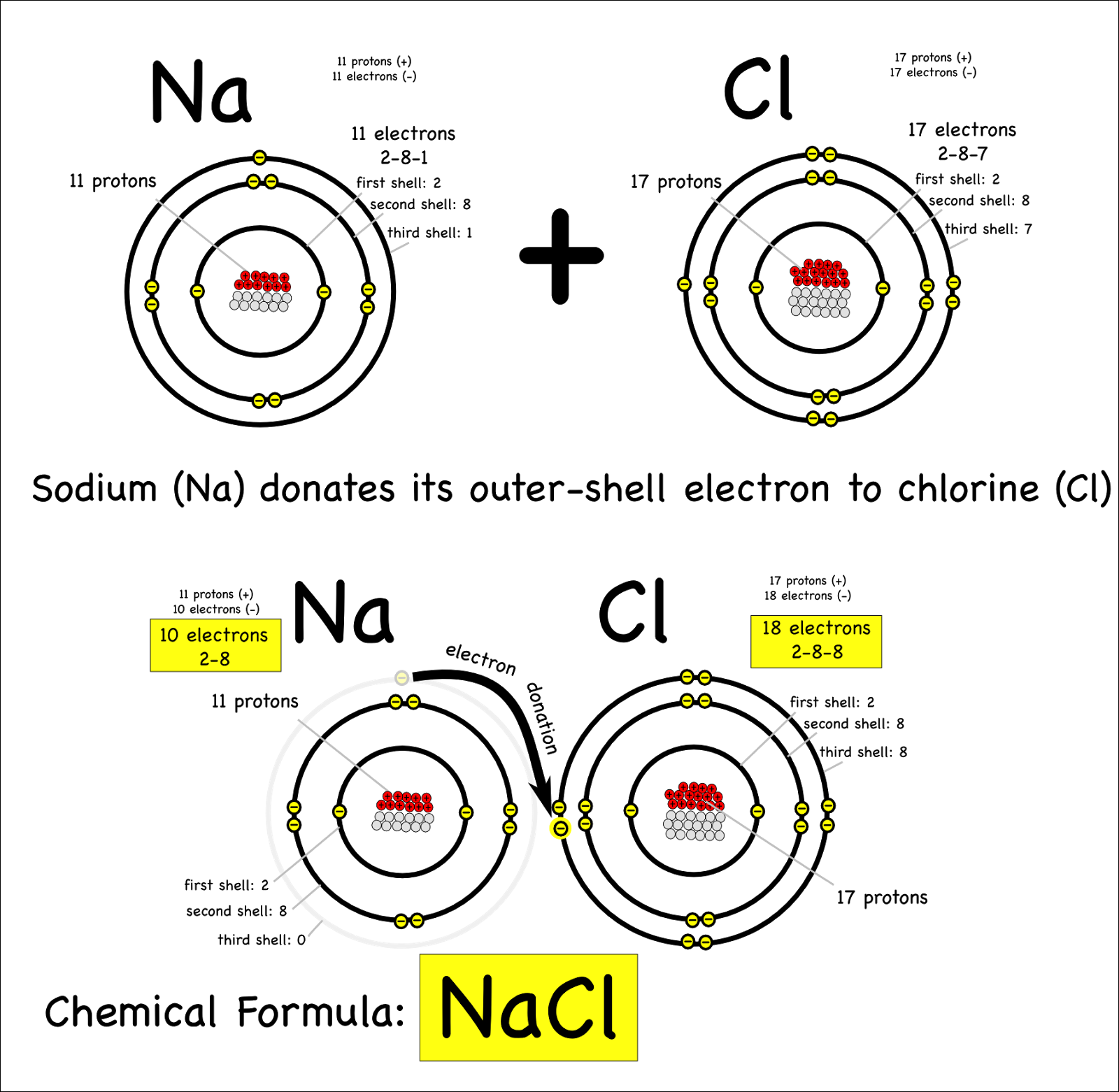
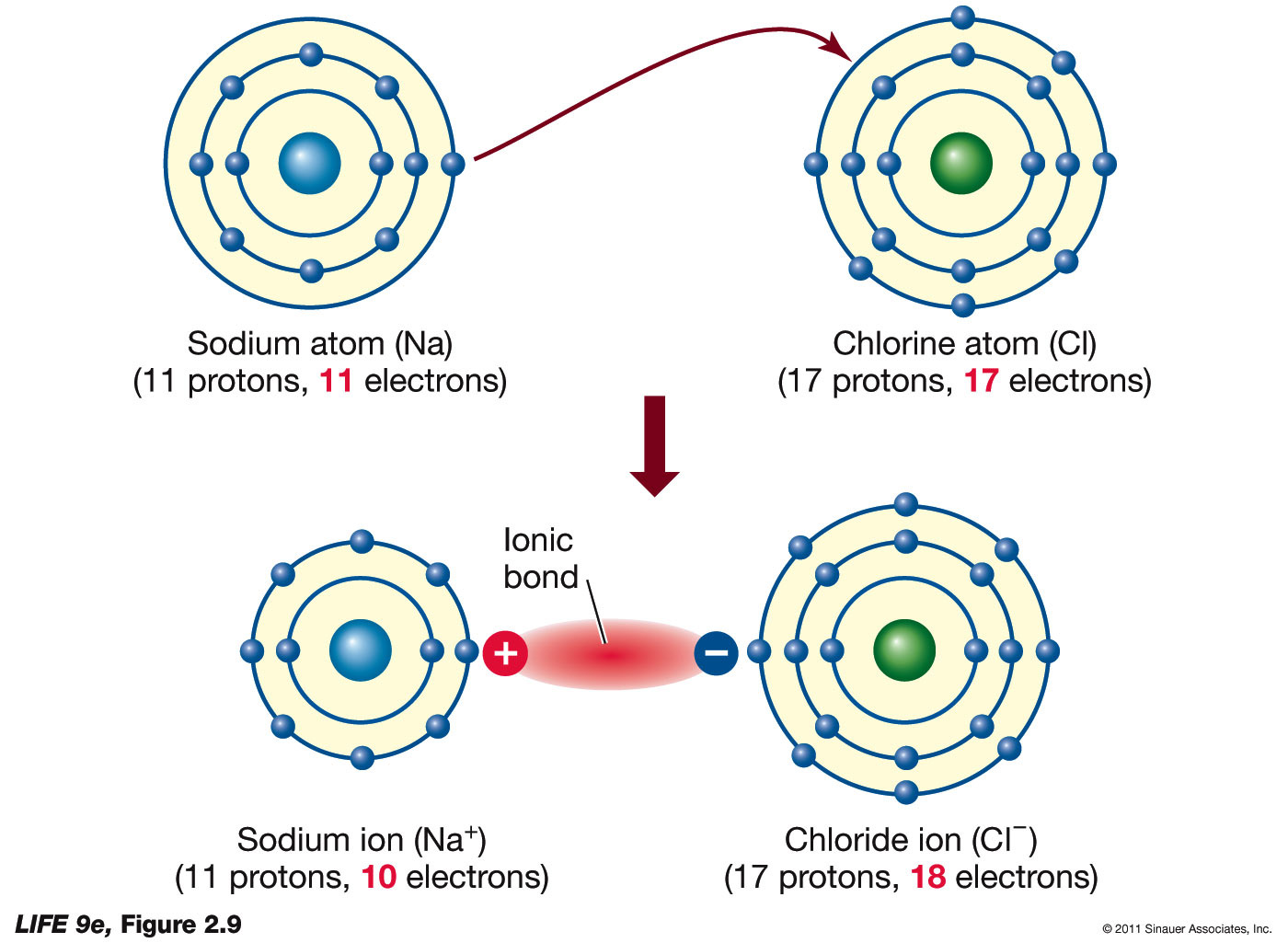
An Ionic Bond Forms Between A - Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. How and why do ionic bonds form? Ionic bonds form between metal atoms and other metal atoms. Check all of the boxes that apply. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Such a bond forms when the valence (outermost) electrons of. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. Ionic bonds form between metal atoms and nonmetal atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge
Check all of the boxes that apply. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge Ionic bonds form between metal atoms and nonmetal atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. How and why do ionic bonds form? Ionic bonds form between metal atoms and other metal atoms.
Ionic bonds form between metal atoms and nonmetal atoms. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bonds form between metal atoms and other metal atoms. Check all of the boxes that apply. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge How and why do ionic bonds form?
Fourth Grade GC August 2013
How and why do ionic bonds form? Such a bond forms when the valence (outermost) electrons of. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Check all of the boxes that apply. Ionic bonds form between metal atoms.
SOLVED An ionic bond forms between what two types of atoms?
Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. How and why do ionic bonds form? Such a bond forms when the valence (outermost) electrons of. An ionic bond forms.
ionic bond Definition, Properties, Examples, & Facts Britannica
Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Such a bond forms when the valence (outermost) electrons of. An ionic.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge Check all of the boxes that apply. Ionic bonds form between metal atoms and nonmetal atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic bond, also known as.
Ionic Bond Definition and Examples
How and why do ionic bonds form? Ionic bonds form between metal atoms and other metal atoms. Check all of the boxes that apply. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. An ionic bond, also known as an electrovalent bond, is a type of chemical bond.
(PPTX) Ionic Nomenclature. An ionic bond forms between metals and
Ionic bonds form between metal atoms and nonmetal atoms. Such a bond forms when the valence (outermost) electrons of. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Check all of the boxes that apply. Ionic bonds form between metal atoms and other metal atoms.
(PPT) Chapter 6 Review. An ionic bond forms between a ___________ and a
Ionic bonds form between metal atoms and other metal atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. How and why do ionic bonds form? Ionic bonds form between metal atoms and nonmetal atoms. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed.
10+ Ionic Bonding Diagram Robhosking Diagram
Check all of the boxes that apply. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Such a bond forms when the valence (outermost) electrons of. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds.
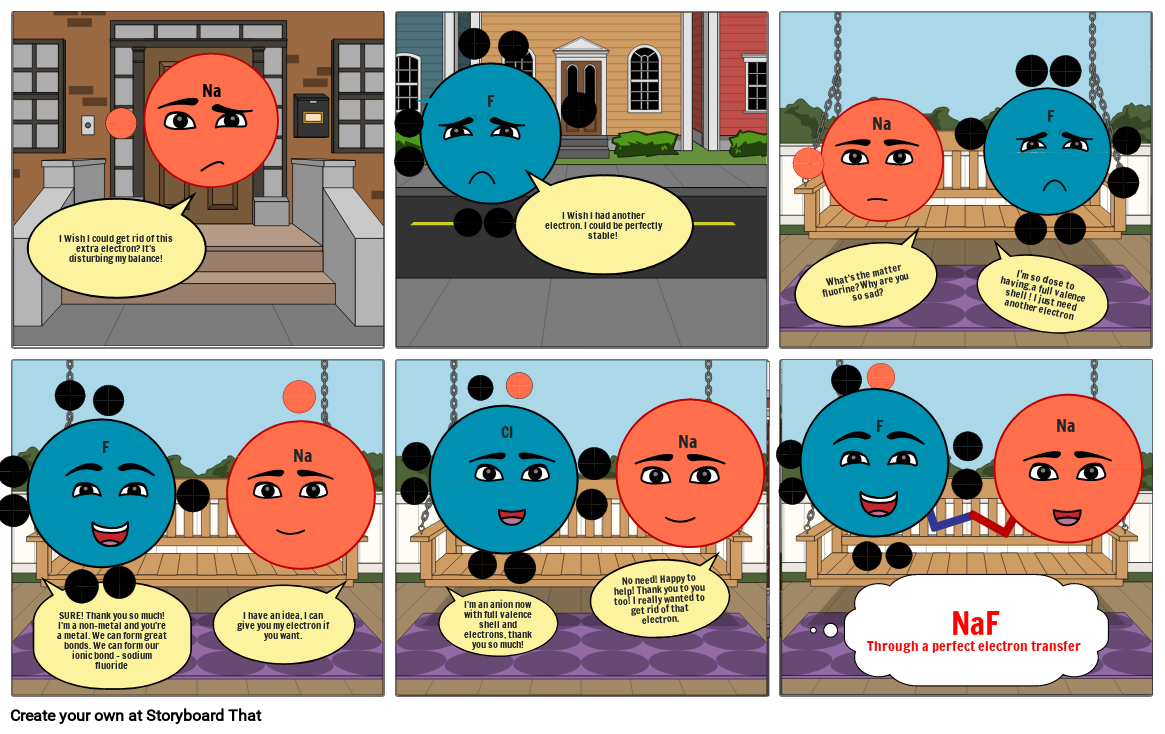
Ionic Bonding Storyboard by cceeeb96
Ionic bonds form between metal atoms and nonmetal atoms. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge Ionic bonds form between metal atoms and other metal atoms..
What Is An Ionic Bond Sciencing Ionic Bonding Ionic Chemical Bond
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge Ionic bonds form between metal atoms and other metal atoms. Ionic bonds form between metal atoms and nonmetal atoms. Check all of the boxes that apply. Such a bond forms when the valence (outermost) electrons of.
Check All Of The Boxes That Apply.
How and why do ionic bonds form? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bonds form between metal atoms and nonmetal atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.
Ionic Bonds Form Between Atoms With Large Differences In Electronegativity, Whereas Covalent Bonds Formed Between Atoms With Smaller Differences In Electronegativity.
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge Such a bond forms when the valence (outermost) electrons of. Ionic bonds form between metal atoms and other metal atoms.






.PNG)