Acids React With Bases To Form Salt And Water
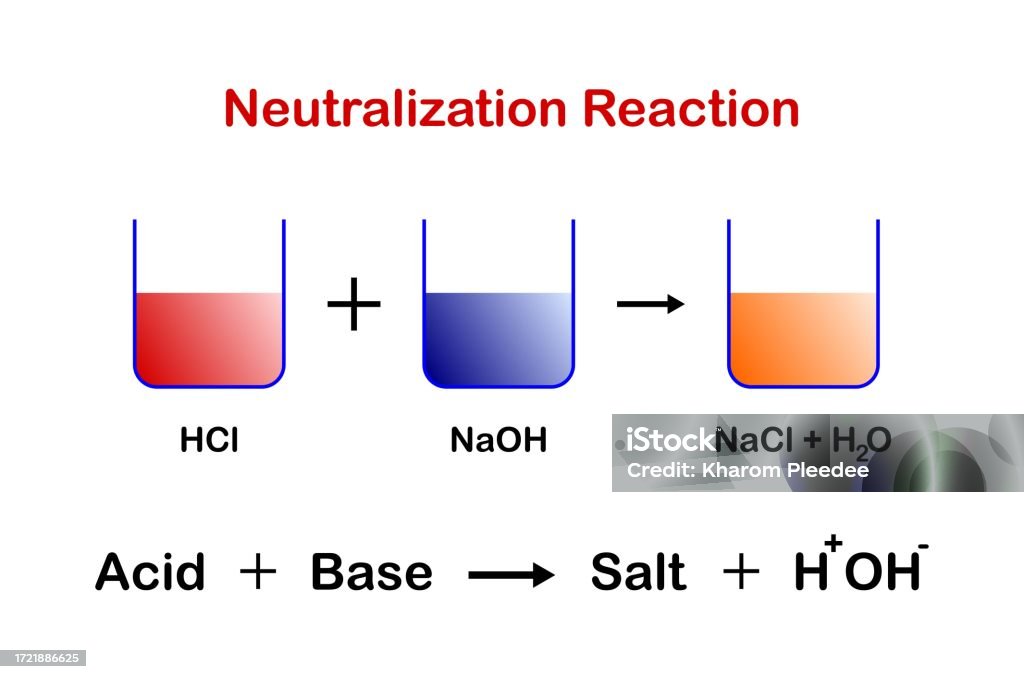
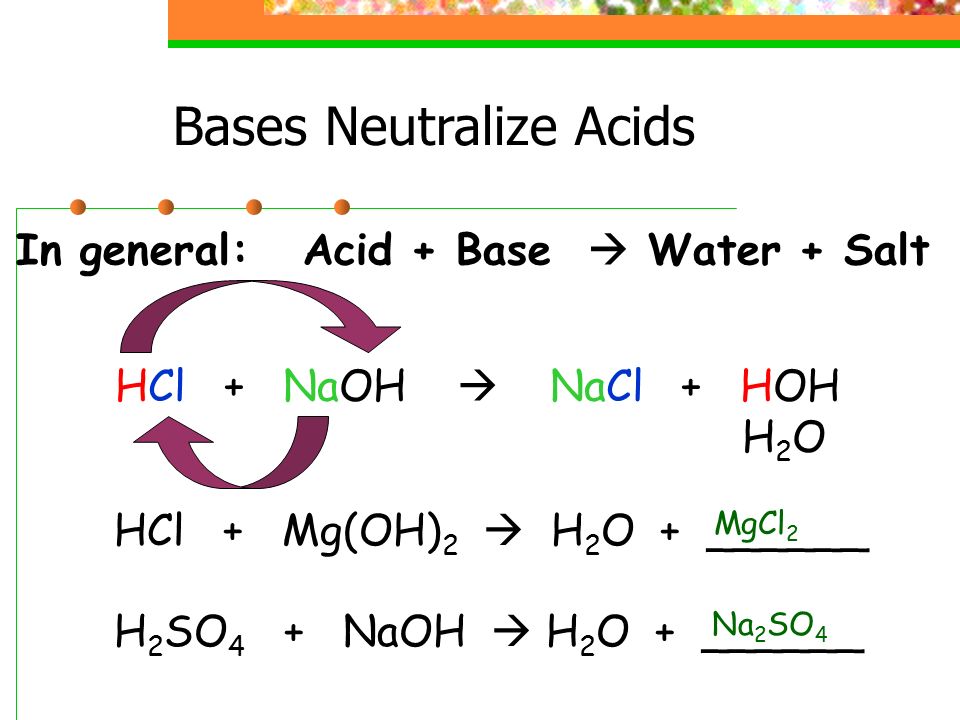
Acids React With Bases To Form Salt And Water - Acid + base → salt + water. Acids react with bases to form a salt and water. When an acid and a base are combined, water and a salt are the products. Acids react with bases in a neutralisation reaction to form salts and water. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. In a neutralisation reaction, an acid and a base combine to form a. Hydrochloric acid reacts with sodium hydroxide to form sodium. When an acid reacts with a base, we get salt and water as products. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. Salts are ionic compounds containing a positive ion other than h+ h + and.
When an acid and a base are combined, water and a salt are the products. Acids react with bases to form a salt and water. Salt solutions do not always. Hydrochloric acid reacts with sodium hydroxide to form sodium. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. Salts are ionic compounds containing a positive ion other than h+ h + and. When an acid reacts with a base, we get salt and water as products. Acid + base → salt + water. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Acids react with bases in a neutralisation reaction to form salts and water.
Acid + base → salt + water. Acids react with bases to form a salt and water. Hydrochloric acid reacts with sodium hydroxide to form sodium. Acids react with bases in a neutralisation reaction to form salts and water. Salt solutions do not always. When an acid reacts with a base, we get salt and water as products. Salts are ionic compounds containing a positive ion other than h+ h + and. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. In a neutralisation reaction, an acid and a base combine to form a. When an acid and a base are combined, water and a salt are the products.
Acids react with bases to form salt and water. This reaction is known as
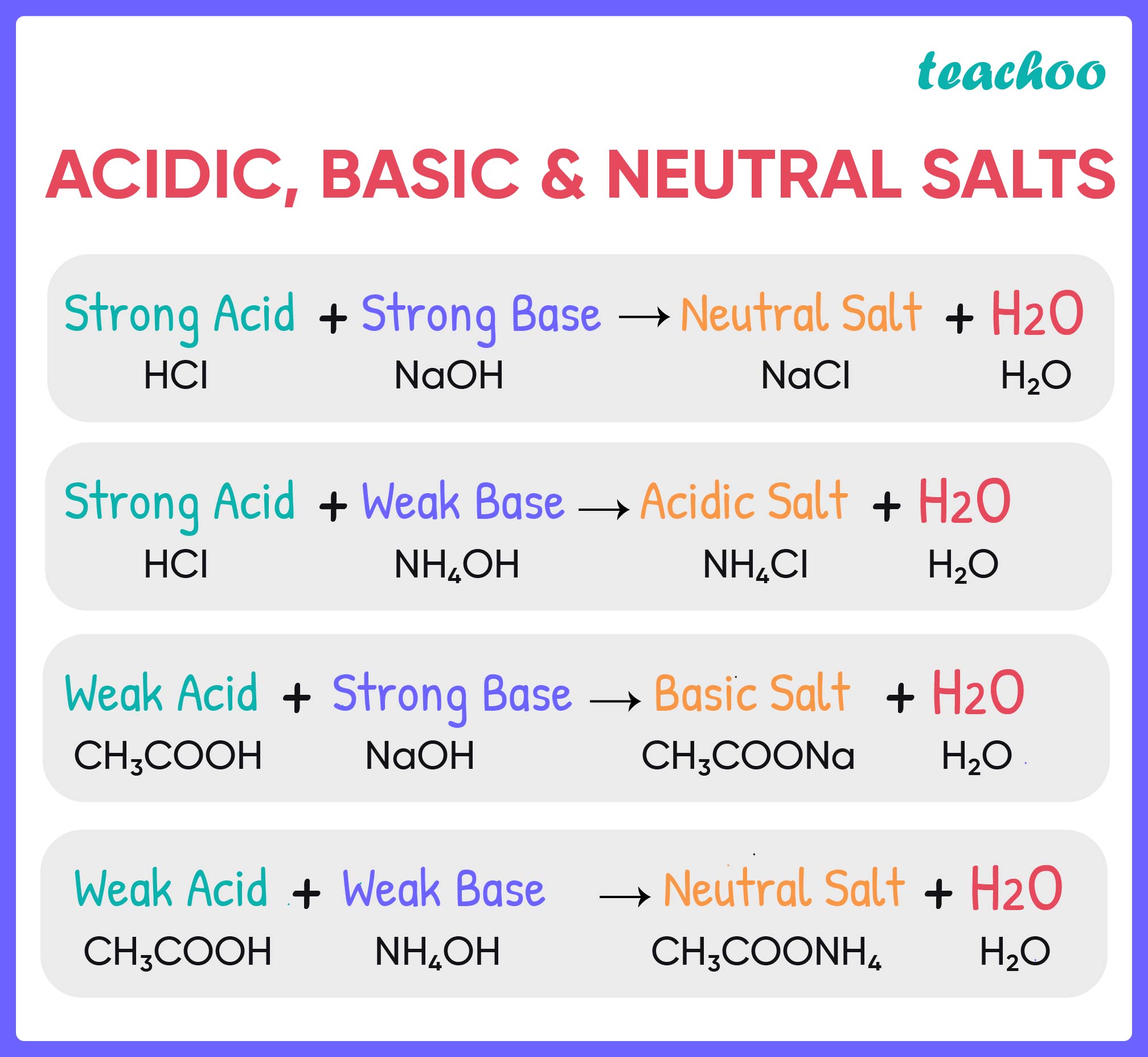
Salt solutions do not always. Salts are ionic compounds containing a positive ion other than h+ h + and. Acids react with bases to form a salt and water. Hydrochloric acid reacts with sodium hydroxide to form sodium. When an acid reacts with a base, we get salt and water as products.
Lesson Plan of Properties and Uses of Acids (Acids, Alkalies and Salts
Hydrochloric acid reacts with sodium hydroxide to form sodium. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. When an acid and a base are combined, water and a salt are the products. Acid + base → salt + water. When an acid reacts with a base, we get salt and water as products.
Acids and it's Properties Definition [with Flowchart and Examples]
Acids react with bases in a neutralisation reaction to form salts and water. Acids react with bases to form a salt and water. When an acid reacts with a base, we get salt and water as products. Acid + base → salt + water. Salts are ionic compounds containing a positive ion other than h+ h + and.
Acids React With Bases To Produce Salt And Water Stock Illustration
When an acid and a base are combined, water and a salt are the products. Salt solutions do not always. Acids react with bases in a neutralisation reaction to form salts and water. When an acid reacts with a base, we get salt and water as products. Hydrochloric acid reacts with sodium hydroxide to form sodium.
Acids and Bases Science with Mrs Beggs
Salts are ionic compounds containing a positive ion other than h+ h + and. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. Hydrochloric acid reacts with sodium hydroxide to form sodium. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Acids react with bases in a neutralisation.
Salts and it's Properties (with Examples) Acids, Bases and Salt
When an acid reacts with a base, we get salt and water as products. Acids react with bases in a neutralisation reaction to form salts and water. Hydrochloric acid reacts with sodium hydroxide to form sodium. Salts are ionic compounds containing a positive ion other than h+ h + and. In a neutralisation reaction, an acid and a base combine.
A Level Chemistry Revision Physical Chemistry Acids And Bases
Salt solutions do not always. When an acid and a base are combined, water and a salt are the products. Acid + base → salt + water. Hydrochloric acid reacts with sodium hydroxide to form sodium. Acids react with bases to form a salt and water.
Acids, Bases and Salts class 7 worksheet witknowlearn Acids bases
Hydrochloric acid reacts with sodium hydroxide to form sodium. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. The acid and base have neutralized each other, and the acidic and basic properties are no longer present. When an acid reacts with a base, we get salt and water as products. Acids react with bases to form a.
Acids, Bases, And Salts Definition, Types, Properties, And, 51 OFF
Hydrochloric acid reacts with sodium hydroxide to form sodium. Salt solutions do not always. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Acids react with bases to form a salt and water. Acids react with bases in a neutralisation reaction to form salts and water.
Acid Base Reaction Examples
Acids react with bases in a neutralisation reaction to form salts and water. When an acid and a base are combined, water and a salt are the products. Acids react with bases to form a salt and water. Salt solutions do not always. Salts are ionic compounds containing a positive ion other than h+ h + and.
Acids React With Bases To Form A Salt And Water.
When an acid and a base are combined, water and a salt are the products. When an acid reacts with a base, we get salt and water as products. Sulfuric acid + copper (ii) oxide → copper (ii) sulfate + water. Acid + base → salt + water.
The Acid And Base Have Neutralized Each Other, And The Acidic And Basic Properties Are No Longer Present.
Acids react with bases in a neutralisation reaction to form salts and water. Salts are ionic compounds containing a positive ion other than h+ h + and. Salt solutions do not always. Hydrochloric acid reacts with sodium hydroxide to form sodium.

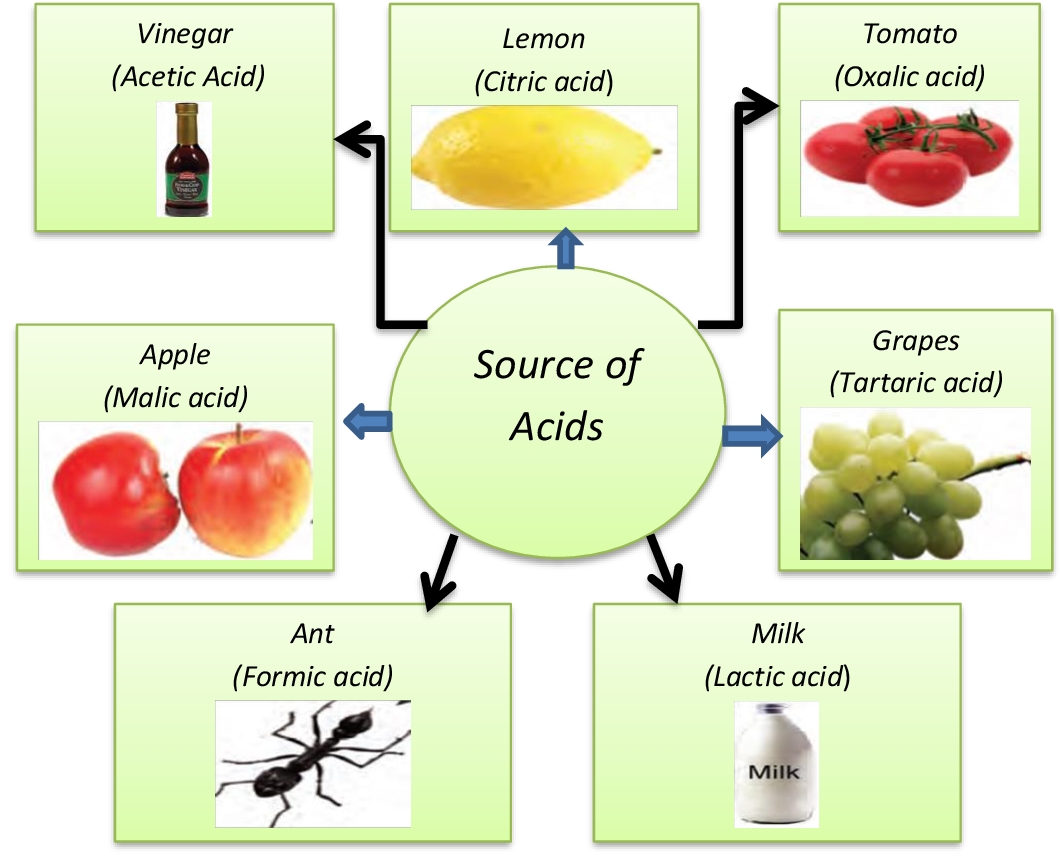
![Acids and it's Properties Definition [with Flowchart and Examples]](https://d1avenlh0i1xmr.cloudfront.net/28568581-671c-4933-be3c-3b6bced35321/some-properties-of-acids-teachoo.jpg)


.png)
